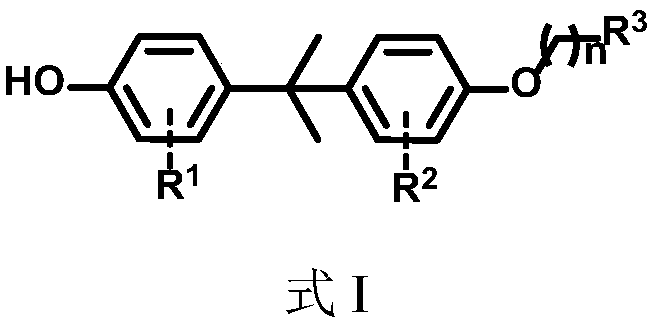
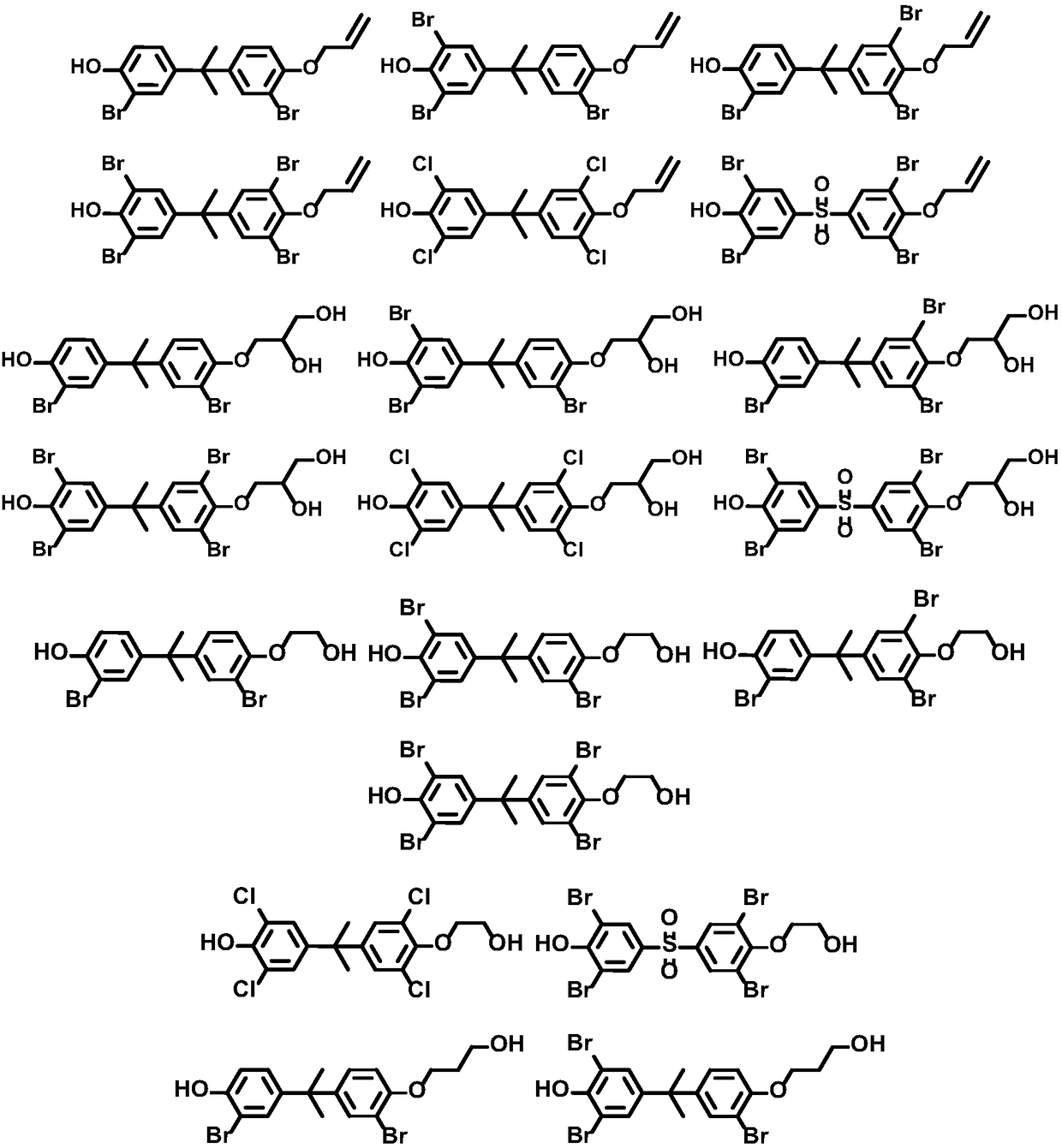
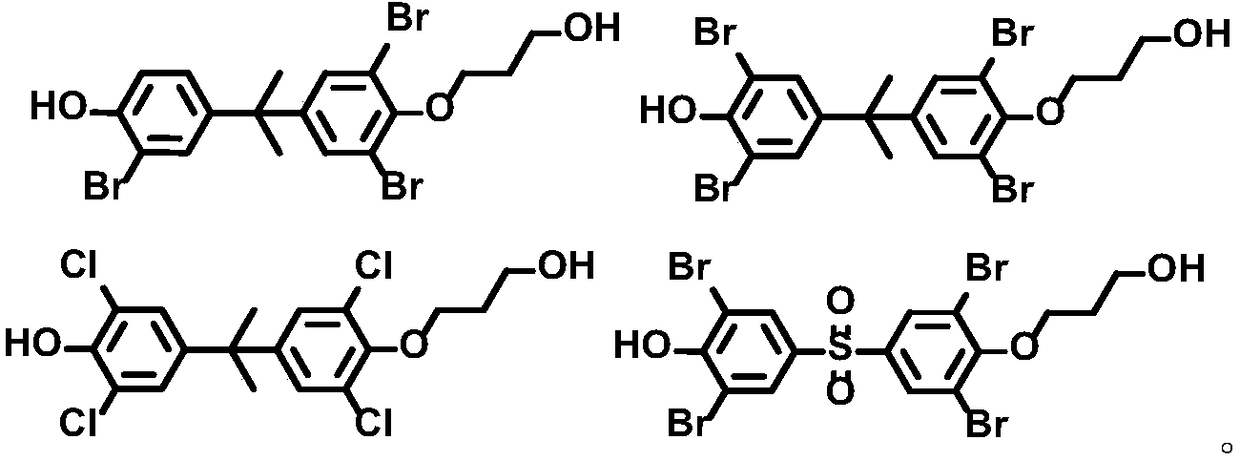
Bisphenol compound monosubstituted fatty alcohol and synthesis method thereof
A compound and oxidant technology, applied in chemical instruments and methods, preparation of organic compounds, dehydration of hydroxyl-containing compounds to prepare ethers, etc., can solve the problems of lack of standard products, lack of synthesis methods, research limitations, etc., to achieve strong applicability and good Atom economy, the effect of avoiding operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. The preparation method of bisphenol compound monosubstituted allyl ether compound
[0056] Mix the halogenated bisphenol shown in formula II with allyl bromide in a solvent, and carry out a substitution reaction in the presence of an alkali solution, and the reaction is completed to obtain the R 3 for -CH=CH 2 When the compound shown in the formula I;
[0057]
[0058] In the formula II, R 1 and R 2 The definition of is the same as that in formula I.
[0059] Experimental procedure: Dissolve bisphenol A compound (1.0 equivalent) shown in formula II in 100ml acetone, add allyl bromide (1.1 equivalent), dropwise add 3mol / L sodium hydroxide aqueous solution (1.8 equivalent), room temperature Stir for 3 hours, adjust the pH of the system to neutral after the reaction is complete, then extract the reactant with dichloromethane, dry it with anhydrous sodium sulfate, and analyze the product point by thin layer chromatography (developing agent is ethyl acetate:petrole...
Embodiment 2
[0148] Embodiment 2, flame retardant performance detection
[0149] With embodiment 1 gained 1.1 compound 4-(2-(4-(allyloxy)-3-bromophenyl)propan-2-yl)-2-bromophenol and 1.4 compound 4-(2-(4-(allyloxy)-3, 5-dibromophenyl)propan-2-yl)-2,6-dibromophenol is prepared as a reactive flame retardant in EPS (polystyrene plastics) and PS (foamed plastics) to prepare corresponding materials, and the materials are carried out Flame retardant performance test. The test results show that the above two compounds added with a mass fraction of 1-5% in polystyrene plastics and foamed plastics are equivalent to the flame retardancy of tetrabromobisphenol A, and the thermal mass loss temperature is: 5-8% (230-240°C), 8-10% (270-280°C), 15-18% (305-320°C).
[0150] The flame retardancy results of the remaining compounds obtained in Example 1 are not substantially different from the above results, and will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com