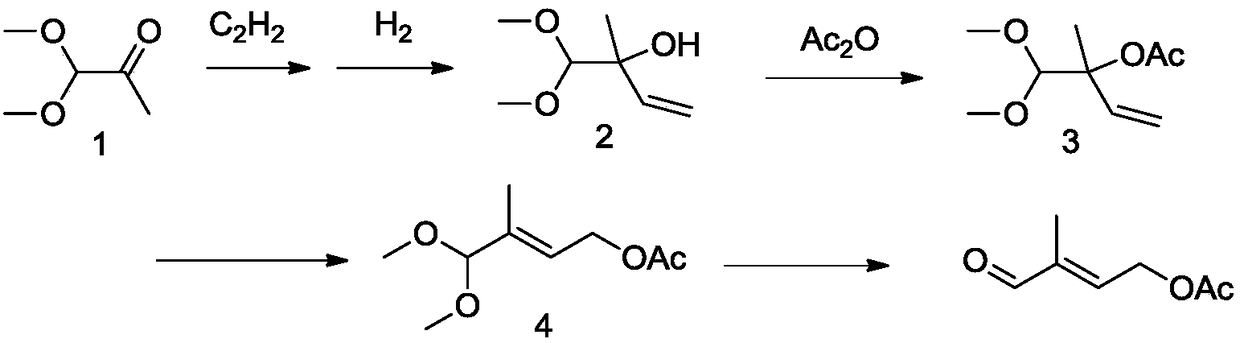
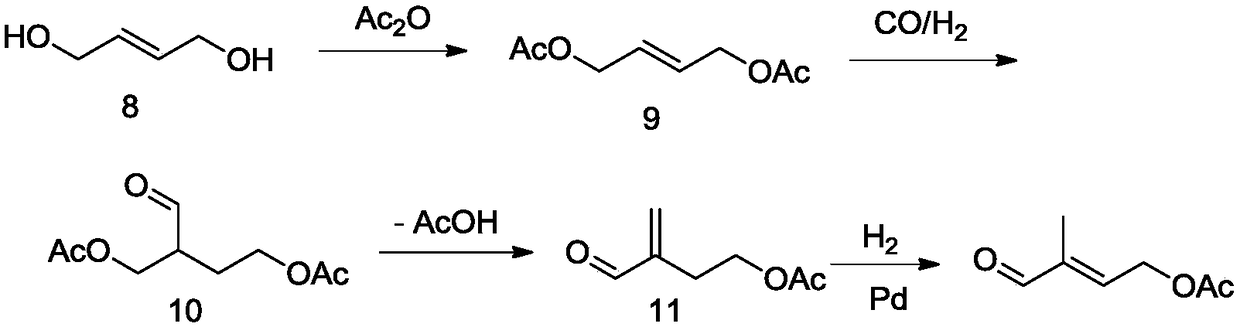
Novel method for preparing 4-acetoxy-2-methyl-2-butenal
A technology of acetoxy and crotonaldehyde, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc., can solve the high requirements for the storage of intermediates and the safety of the reaction, and a large number of by-products. , low yield and other problems, to achieve the effect of easy to achieve industrial production, mild reaction conditions and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add 2500g of methanol, 500g of tiglic aldehyde, and 0.5g of p-toluenesulfonic acid into a 5L reactor, stir, raise the temperature to 65°C, and reflux for 1h. After the reaction was completed, it was cooled to room temperature. After the reaction solution is adsorbed by the basic resin to remove the catalyst, the methanol is rectified to obtain the acetal (I) with a yield of 98%.
[0059] Add 1040g of acetonitrile, 260g of acetal (I), 2g of cuprous chloride, and 32g of 2,2'-bipyridine into a 2L three-necked flask. Stir, cool to 5°C, add dropwise 528g of tert-butyl peroxyacetate solution, and control the temperature of the reaction solution to 0°C. After the dropwise addition was completed, the reaction was continued at 0°C for 1 h. After the reaction was finished, samples were taken for gas phase analysis, and the yield was calculated to be 86% according to the analysis results. The acetal ester (II) was obtained by rectification and separation with a purity of >99%. ...
Embodiment 2
[0063] Add 2500g methanol, 250g tiglic aldehyde, 2.5g LaCl in the 5L reactor 3 , stirred, heated to 65°C, and refluxed for 5h. After the reaction was completed, it was cooled to room temperature. After the reaction solution was adsorbed by the basic resin to remove the catalyst, the methanol was rectified to obtain the acetal (I) with a yield of 97%.
[0064] Add 1560g of DMF, 260g of acetal (I), 17.4g of cuprous trifluoromethanesulfonate, and 14.6g of 1,10-phenanthroline into a 5L three-necked flask. Stir, adjust the temperature to 20°C, add dropwise 1056g of tert-butyl peroxyacetate solution, and control the temperature of the reaction solution to 20°C. After the dropwise addition was completed, the reaction was continued at 20°C for 5h. After the reaction was completed, samples were taken for gas phase analysis, and the yield was calculated to be 89% according to the analysis results. The acetal ester (II) was obtained by rectification and separation with a purity of >9...
Embodiment 3
[0067] Add 1500g methanol, 250g tiglic aldehyde, 1.25g ZrCl in the 5L reactor 4 , stirred, heated to 65°C, and refluxed for 2h. After the reaction was completed, it was cooled to room temperature. After the reaction solution was adsorbed by the basic resin to remove the catalyst, the methanol was rectified to obtain the acetal (I) with a yield of 97%.
[0068] Add 1300g of tetrahydrofuran, 260g of acetal (I), 14.8g of copper trifluoromethanesulfonate, and 28.4g of terpyridine into a 5L three-necked flask. Stir, cool to 10°C, add dropwise 792g of tert-butyl peroxyacetate solution, and control the reaction temperature to 10°C. After the dropwise addition was completed, the reaction was continued at 10°C for 2h. After the reaction was completed, samples were taken for gas phase analysis, and the yield was calculated to be 90% according to the analysis results. The acetal ester (II) was obtained by rectification and separation with a purity of >99%.
[0069] Add 1150g of wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com