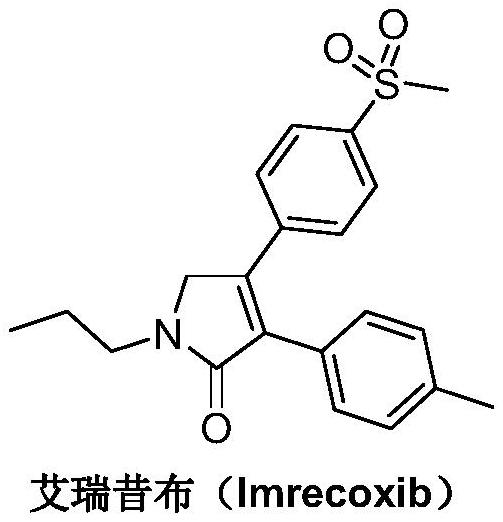
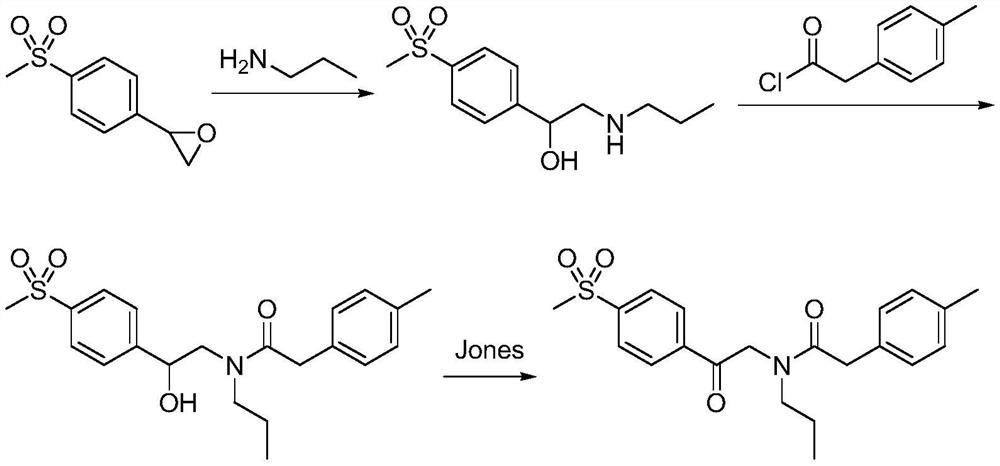
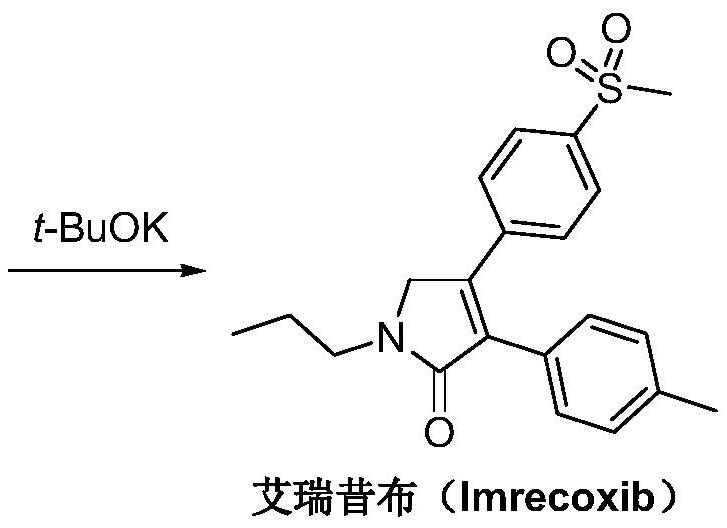
The synthetic method of Erecoxib
A synthesis method and n-propylamino technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of many impurities and by-products, unfavorable post-processing and purification, and difficulty in meeting the quality requirements of raw materials, and achieve the effects of less impurities and lower costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A) Synthesis of 2-n-propylamino-1-p-methylsulfonyl acetophenone:
[0032] N-n-propyl-β-hydroxy-4-methylsulfonylphenethylamine (14.0g) was dissolved in 1,2-dichloroethane (280mL), potassium permanganate (9.5g) and water (1080mL) were added , raised to 20°C and reacted for 8 hours until the reaction was complete. After post-treatment, the obtained crude product was recrystallized with ethanol to obtain 2-n-propylamino-1-p-methylsulfonylacetophenone, off-white solid 12.4g, yield 89 %.
[0033] B) Synthesis of Erecoxib:
[0034] Dissolve 2-n-propylamino-1-p-methylsulfonylacetophenone (10.0g) in isopropanol (200mL), add sodium isopropoxide (6.4g), stir and cool to 5-10°C in an ice bath, Add a solution of ethyl p-tolyl acetate (10.5g) in isopropanol (15mL) dropwise, rise to 20°C and react for 12h until the reaction is complete. After post-treatment, the obtained crude product is recrystallized with ethanol to obtain Erecoxib, white Solid 13.6g, yield 94%.
Embodiment 2
[0036] A) Synthesis of 2-n-propylamino-1-p-methylsulfonyl acetophenone:
[0037] Dissolve N-propyl-β-hydroxy-4-methylsulfonylphenethylamine (27.0g) in acetonitrile (500mL), add hydrogen peroxide (0.105mol) and water (1500mL), raise the temperature to 40°C for 4h until the reaction is complete , after post-treatment, the resulting crude product was recrystallized from ethanol to obtain 2-n-propylamino-1-p-methylsulfonylacetophenone, 25.4 g of an off-white solid, with a yield of 95%.
[0038] B) Synthesis of Erecoxib:
[0039] Dissolve 2-n-propylamino-1-p-methylsulfonylacetophenone (22.0g) in ethanol (450mL), add sodium ethoxide (10.6g), stir and cool in an ice bath to 5-10°C, add p-toluene dropwise Methyl acetate (18.4g) in ethanol (25mL) was raised to 70°C for 9h until the reaction was complete. After post-treatment, the resulting crude product was recrystallized with ethanol to obtain Erecoxib, a white solid of 30.2g, yield 95%.
Embodiment 3
[0041] A) Synthesis of 2-n-propylamino-1-p-methylsulfonyl acetophenone:
[0042] Dissolve N-propyl-β-hydroxy-4-methylsulfonylphenethylamine (100.0g) in ethanol (1800mL), add pyridinium chlorochromate (96.3g) and water (3900mL), and heat up to 60°C for reaction After 2 hours to complete the reaction, after post-treatment, the obtained crude product was recrystallized from ethanol to obtain 2-n-propylamino-1-p-methylsulfonylacetophenone, 92.3 g of off-white solid, with a yield of 93%.
[0043] B) Synthesis of Erecoxib:
[0044] 2-n-propylamino-1-p-methylsulfonylacetophenone (91.5g) was dissolved in tetrahydrofuran (1500mL), sodium hydroxide (21.5g) was added, stirred and cooled to 5-10°C in an ice bath, and p- A solution of ethyl tolyl acetate (70.3g) in tetrahydrofuran (125mL) was raised to 90°C for 6h until the reaction was complete. After post-treatment, the obtained crude product was recrystallized with ethanol to obtain Erecoxib, a white solid of 125.8g. The rate is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com