Synthesis method of triphenyl-n-(colchicine amido) butylphosphonium chloride and its application in antitumor drugs
A technology of narciscine amide and triphenyl chloride, which is applied in the synthesis field of triphenyl-n-butylphosphonium chloride, can solve the problems of high toxicity of colchicine, limited application, animal death, etc., and achieves Improve the efficiency of drug action, reduce the dosage, and overcome the effect of excessive toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
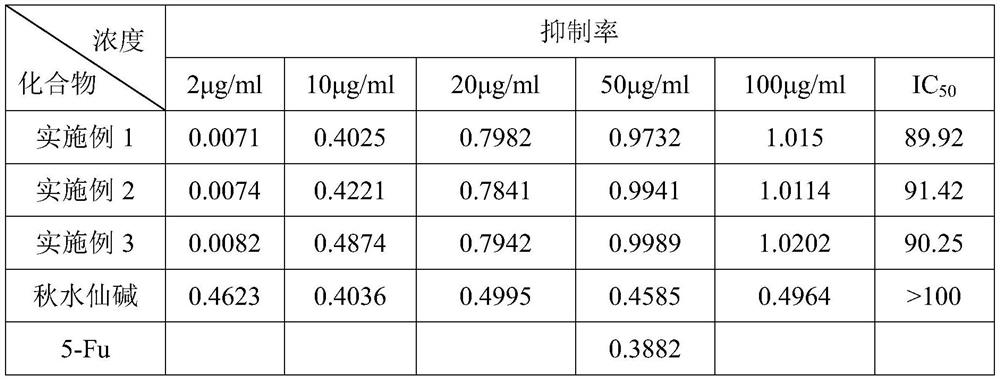
Embodiment 1
[0041] A kind of synthetic method of triphenyl chloride-4-(colchicine amido) butylphosphonium compound of the present embodiment comprises the steps:
[0042] (1) Synthesis of N-Boc colchicine
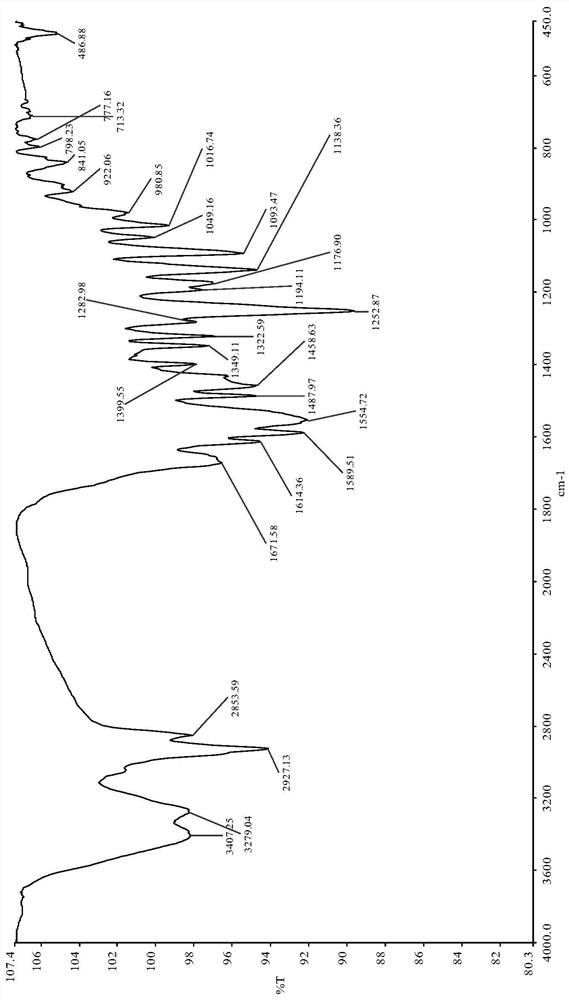
[0043] Dissolve 1.04g (2.6mmol) colchicine in 15ml of dichloromethane solution after dry distillation, then add 0.35g (2.91mmol) 4-dimethylaminopyridine, 0.42ml (2.91mmol) to the three-necked flask successively triethylamine. Stir and reflux and slowly dropwise add 3.20 g (14.66 mmol) of Boc anhydride to the solution, react for 6 h, and monitor the progress of the reaction by TLC. After the reaction was complete, the solvent was distilled off under reduced pressure and separated by column chromatography to obtain 0.93 g of a light yellow solid with a yield of 68.72%. m.p.: 87.3—89.6°C. IR(KBr)cm -1 :3653.34,2974.62,2934.58,2836.21,1737.32,1679.84,1618.21,1588.59,1488.51,1460.13,1435.05,1407.35,1370.32,1324.04,1283.67,1252.04,1141.28,1092.32,1021.12,1001.58,982.51,923.07,846.03,774....
Embodiment 2
[0053] A kind of synthetic method of triphenyl chloride-3-(colchicine amido) butylphosphonium compound of the present embodiment is basically the same as Example 1, the difference is only in:
[0054] (1) the chloroalkyl chloride that the present embodiment adopts is 3-chlorobutyryl chloride;
[0055] (2) The process of step (4) and step (5) is different:
[0056] (4) Synthesis of N-3-chlorobutyryl deacetylcolchicine
[0057] Take 0.50 g of deacetylcolchicine prepared in step (3), dissolve 0.15 ml of triethylamine in 10 ml of chloroform, stir in an ice-water bath, and add 0.15 ml of 3-chlorobutyryl chloride dropwise into the solution. After reacting for 2 hours, remove the ice-water bath and heat to condense to reflux, and react for 1.5 hours. The progress of the reaction was monitored by TLC. After the reaction was complete, the pH was adjusted to neutral with triethylamine, and the solvent was distilled off under reduced pressure. Column chromatography separated to obtai...
Embodiment 3
[0061] A kind of synthetic method of triphenyl chloride-2-(colchicine amido) butylphosphonium compound of the present embodiment is basically the same as Example 1, the difference is only in:
[0062] (1) the chloroalkyl chloride that present embodiment adopts is 2-chlorobutyryl chloride;
[0063] (2) The process of step (4) and step (5) is different:
[0064] (4) Synthesis of N-2-chlorobutyryl deacetylcolchicine
[0065] Take 0.50 g of deacetylcolchicine prepared in step (3), dissolve 0.125 ml of triethylamine in 10 ml of chloroform, stir in an ice-water bath, and add 0.125 ml of 2-chlorobutyryl chloride dropwise into the solution. After reacting for 2 hours, remove the ice-water bath, heat and condense to reflux, and react for 2 hours. The progress of the reaction was monitored by TLC. After the reaction was complete, the pH was adjusted to neutral with triethylamine, and the solvent was distilled off under reduced pressure. Separated by column chromatography, 0.38 g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com