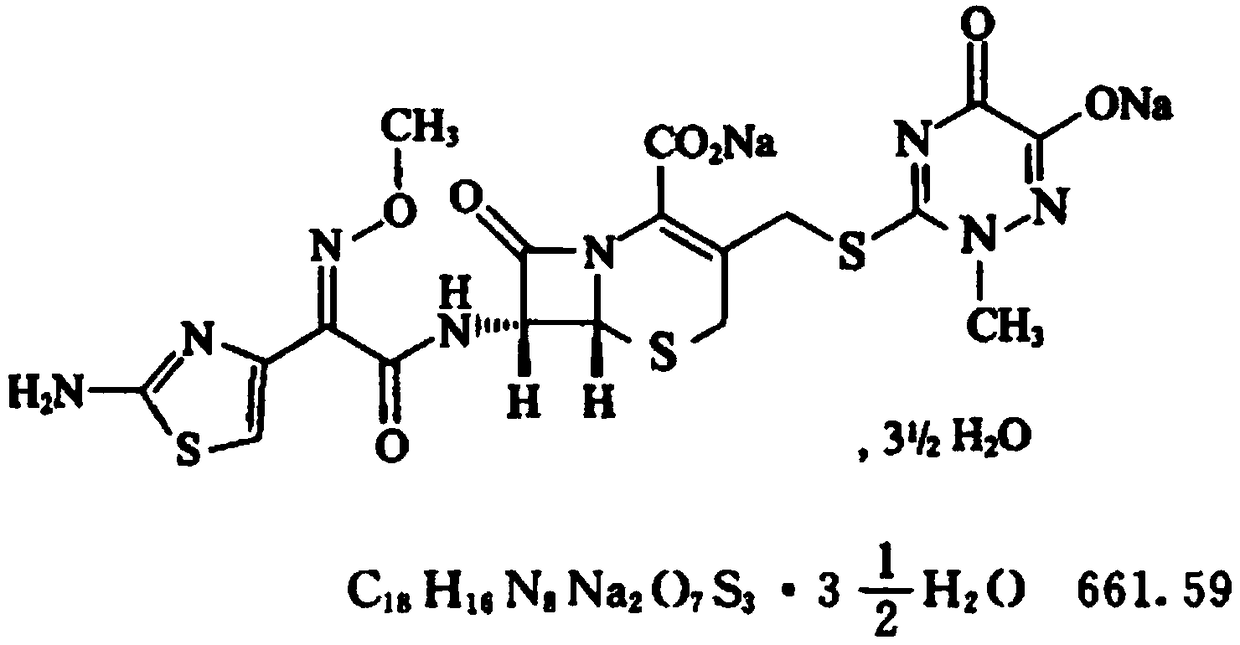
Preparation method of ceftriaxone sodium powder preparation for injection
A technology for ceftriaxone sodium and injection, applied in the field of medicine, can solve problems such as poisoning of production operators and surrounding environment, reduce product purity, aggravate side effects, etc., and achieve the effects of not easy oxidative degradation, good crystal fluidity, and improved quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of ceftriaxone sodium powder preparation for injection, comprising the following steps:
[0030] A. First add the mixed solution of solvent a and water to the reaction tank 1, control the temperature in the reaction tank 1 to be 5-10°C, stir and add the crude product of ceftriaxone sodium and stabilizer until the dissolution is complete, then add the decolorizing agent and filter aid, filter after decolorization to obtain filter cake and filtrate, and finally wash the filter cake with mixed solution to obtain lotion, and the lotion and filtrate are combined to obtain the crude product solution of ceftriaxone sodium, which is then transferred to reaction tank 2; wherein solvent a is methanol or Any one of ethanol, the volume of solvent a in milliliters, the volume of water in milliliters and the weight in grams of crude ceftriaxone sodium are in a ratio of 1 to 2:10 to 20:5; the stabilizer is sodium sulfite and sodium bisulfite Or any one in citric aci...
Embodiment 1
[0047] A preparation method of ceftriaxone sodium powder preparation for injection, comprising the following steps:
[0048] A. Add a mixed solution consisting of 2mL of methanol and 20mL of water to the reaction tank 1, stir well, reserve 2mL of the mixed solution as a washing solution, control the temperature of the reaction tank 1 at 5°C to 6°C, stir and add 0.1g of sodium sulfite and ceftriaxone sodium Crude product 10g is dissolved completely; Add activated carbon 0.5g, filter aid perlite 0.5g, filter after 0.5h of decolorization, wash the filter cake with 2mL lotion, the lotion and filtered filtrate are combined to be ceftriaxone sodium crude product solution, Then transfer to reaction tank 2;
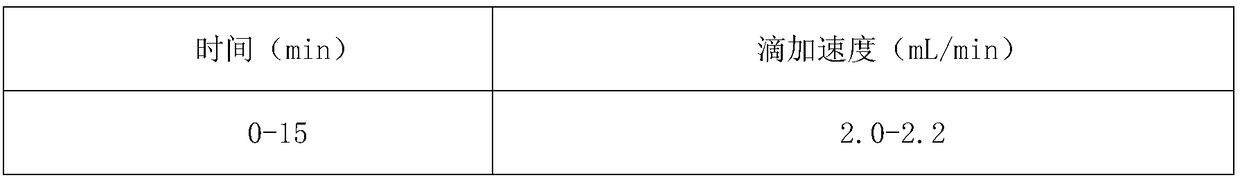
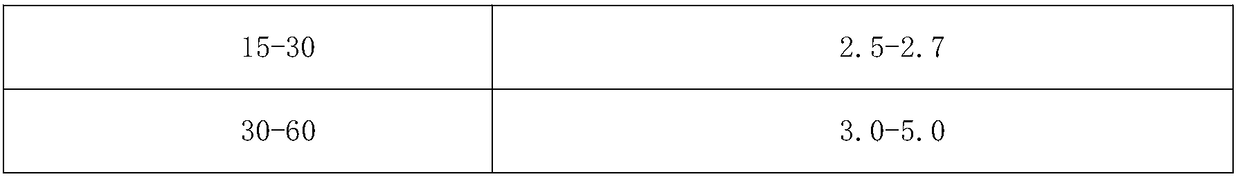
[0049] B. Add 100 mL of acetone to the crystallization tank, control the temperature of the crystallization tank at 15°C to 16°C, control the stirring speed at 150 to 160 rpm, and add the crude ceftriaxone sodium solution in the reaction tank 2 dropwise; add the drop rate accordi...
Embodiment 2
[0058] A preparation method of ceftriaxone sodium powder preparation for injection, comprising the following steps:
[0059] A. Add a mixed solution consisting of 8mL of ethanol and 80mL of water to the reaction tank 1, stir well, reserve 8mL of the mixed solution as a washing liquid, control the temperature of the reaction tank 1 at 9°C to 10°C, stir and add 1g of sodium bisulfite, cephalosporin Trixone sodium crude product 20g to dissolve; Add activated carbon 2g, filter aid diatomite 2g, filter after decolorization 1h, wash the filter cake with 8mL washing liquid, the washing liquid and the filtrate obtained by filtering are combined to be ceftriaxone sodium crude product solution, Then transfer to reaction tank 2;
[0060] B. Add 400 mL of acetonitrile to the crystallization tank, control the temperature of the crystallization tank at 24°C to 25°C, control the stirring speed at 190 to 200 rpm, and add the crude ceftriaxone sodium solution in the reaction tank 2 dropwise; a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com