A sustained-release hypoglycemic pharmaceutical composition and its preparation method and application
A hypoglycemic and slow-release technology, which is applied in drug combination, drug delivery, and pharmaceutical formulations, can solve problems such as side effects and reduce patient compliance, and achieve improved hypoglycemic effect, improved medication compliance, and excellent hypoglycemic effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
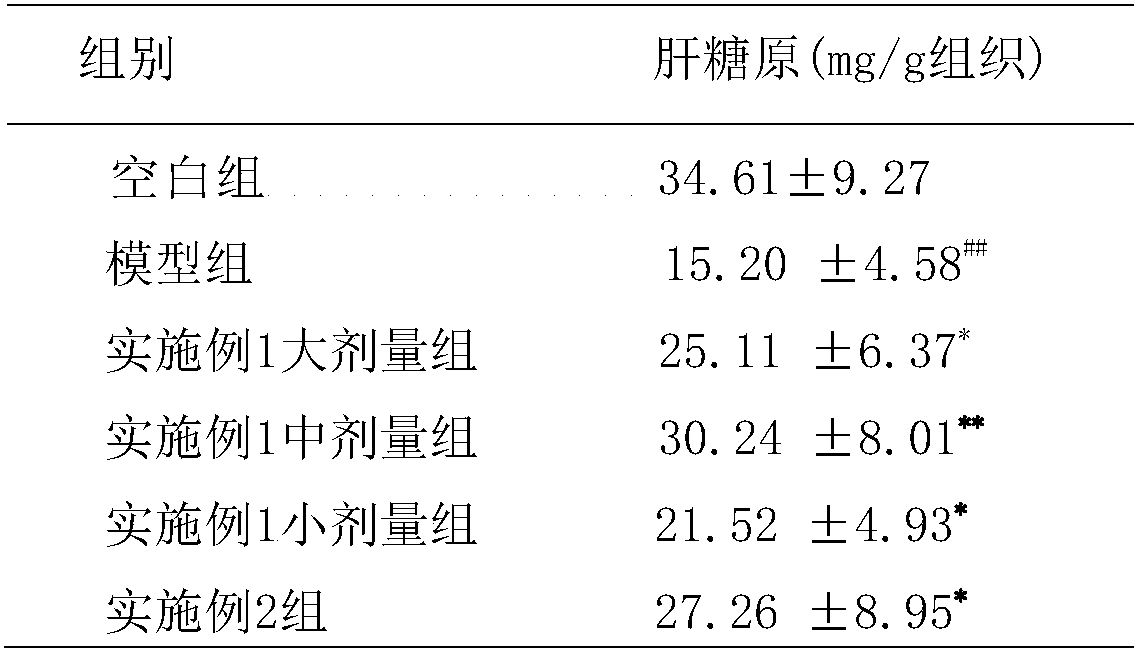
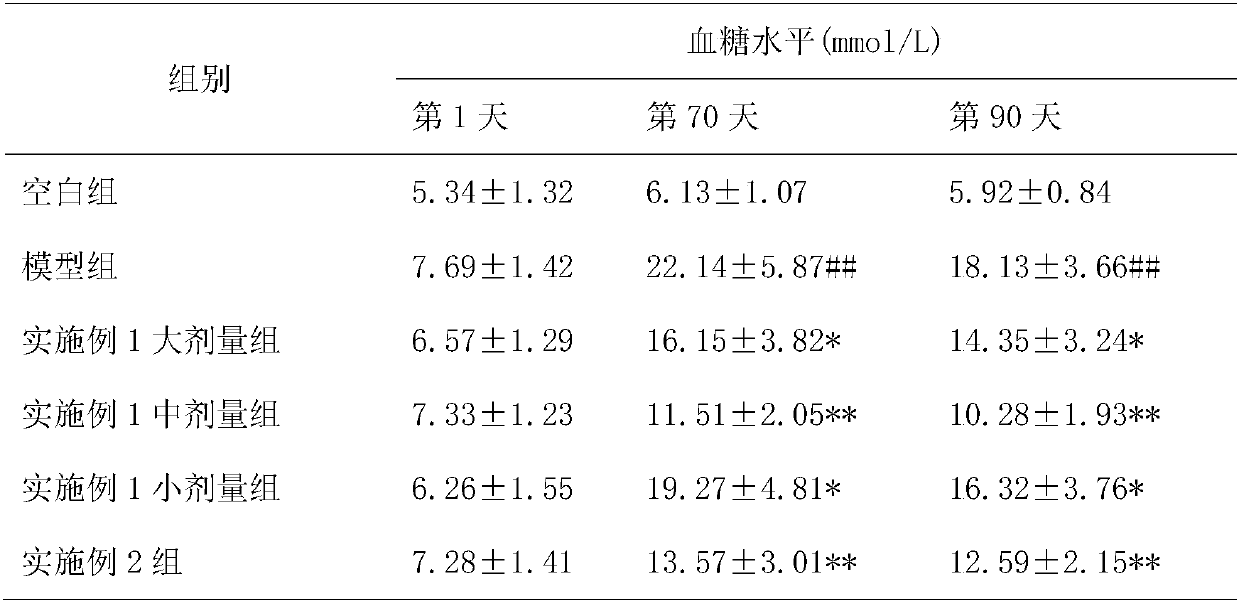
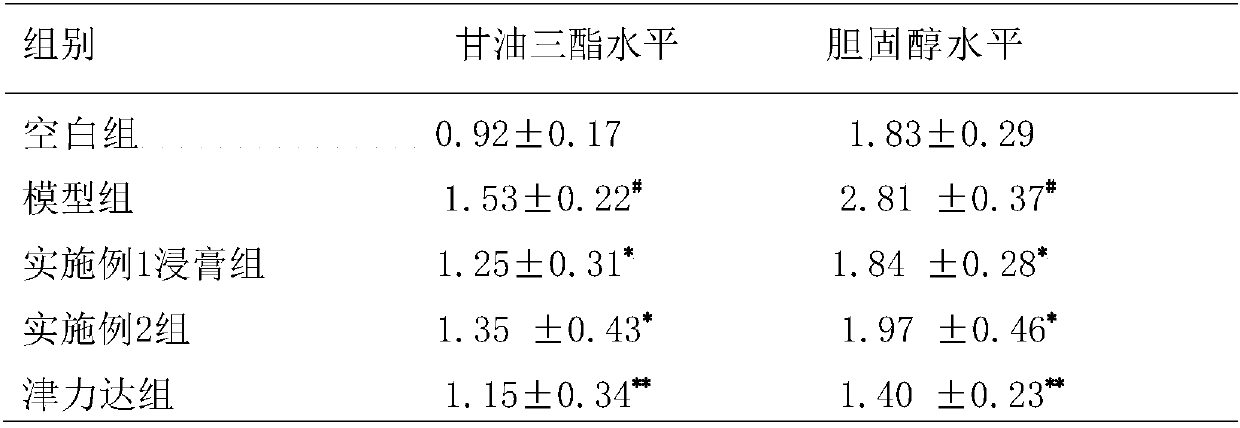
[0049] Embodiment 1: A kind of slow-release tablet for lowering blood sugar
[0050] Rehmannia 5g, Yam 4g, Cornus 4g, Danpi 3g, Poria 3g, Alisma 3g, Lychee 2.5g, Atractylodes 2.5g, Ghost Arrow Feather 1.5g, Scrophulariaceae 3g, Epimedium 2.5g, Cistanche 2.5g , black aconite 0.5g, cinnamon 0.8g, gallinacea 2g, ephedra 0.5g, prepared according to the following method:
[0051] (1) material preparation: take each raw material according to the formula;
[0052] (2) Pulverization: Cistanche deserticola and gallinacea are pulverized through a 30-mesh sieve to obtain powder, which is set aside;
[0053] (3) Extraction: Add 80% of the total amount of 4 times the total amount of raw land, yam, cornus, paeonol, Poria cocos, Alisma, lychee core, herb, ghost arrow feather, Scrophulariaceae, epimedium, black aconite, cinnamon, and ephedra. % ethanol, soaked for 1.5h, then refluxed for 1.5h, filtered to obtain the filtrate for later use, fully used 3 times of 80% ethanol to reflux extract...
Embodiment 2
[0055] Embodiment 2: A kind of slow-release hypoglycemic capsule
[0056] Rehmannia 5g, Yam 4g, Cornus 4g, Danpi 3g, Poria 3g, Alisma 3g, Lychee 2.5g, Atractylodes 2.5g, Ghost Arrow Feather 1.5g, Scrophulariaceae 3g, Epimedium 2.5g, Cistanche 2.5g , black aconite 0.5g, cinnamon 0.8g, gallinacea 2g, ephedra 0.5g, prepared according to the following method:
[0057] (1) material preparation: take each raw material according to the formula;
[0058] (2) Pulverization: Cistanche deserticola and gallinacea are pulverized through a 30-mesh sieve to obtain powder, which is set aside;
[0059] (3) Extraction: Add 80% of the total amount of 4 times the total amount of raw land, yam, cornus, paeonol, Poria cocos, Alisma, lychee core, herb, ghost arrow feather, Scrophulariaceae, epimedium, black aconite, cinnamon, and ephedra. % ethanol, soaked for 1.5h, then refluxed for 1.5h, filtered to obtain the filtrate for later use, fully used 3 times of 80% ethanol to reflux extracted the filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com