Antimicrobial peptide cecropin A mutant and coding gene, preparation method and application thereof
A technology of peptide cecropin and mutants, applied in the fields of genetic engineering and biopharmaceuticals, can solve the problems of weak antibacterial performance, achieve strong antibacterial performance, good non-hemolytic properties, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the gene design of antimicrobial peptide PEW300
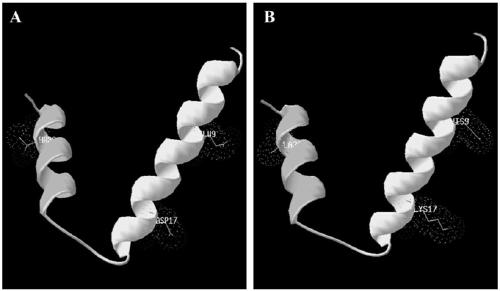
[0044] (1) According to the amino acid sequence composition, charge and hydrophobicity analysis of the natural antimicrobial peptide cecropin A, amino acids in non-conserved regions were selected for amino acid mutation. In order to improve the positive charge of the N-terminus and the hydrophobicity of the C-terminus of the antimicrobial peptide, the Glu at the 9th position was replaced with His, the Asp at the 17th position was replaced with Lys, and the Thr at the 33rd position was replaced with Ala. The amino acid sequence composition of the finally designed antimicrobial peptide is shown in SEQ ID No:3. The physical and chemical properties of the natural antimicrobial peptide cecropin A and the mutant peptide PEW300 are shown in Table 1.
[0045] (2) Through the homology modeling of the mutant peptide PEW300, and the analysis of the electron cloud and hydrophobicity analysis at the mutation site, it c...
Embodiment 2
[0046] Example 2: Construction of recombinant expression vector pET-pew300
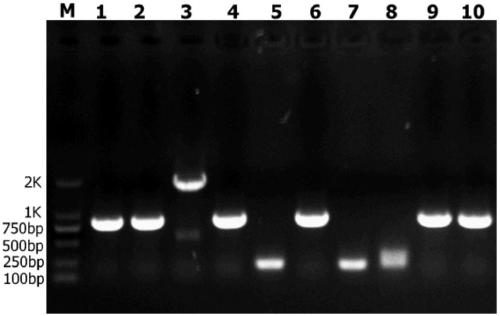
[0047] (1) According to the gene sequence of the self-aggregating short peptide ELK16, the self-cleaving short peptide Mxe GyrA and the linking peptide PT linker (PTPPTTPTPPTTPTPTP), the corresponding DNA sequence Mxe-PTlinker-ELK16 was synthesized at Gene Synthesis Company (Shanghai Sangong), and synthesized Two enzyme cleavage sites, Nde I and Xho I, were introduced into the upstream and downstream of the Mxe-PT linker-ELK16 gene fragment respectively. Then, the pET30a(+) plasmid and the Mxe-PT linker-ELK16 gene fragment were double-digested respectively, and connected to form the plasmid pET-Mxe-PTlinker-ELK16.
[0048] (2) According to the gene sequence of the antimicrobial peptide, its DNA sequence PEW300 was synthesized in Gene Synthesis Company (Shanghai Sangong), and the PEW300 gene was synthesized into pUC57 plasmid to form pUC57-PEW300 plasmid. Design the following primers (PEW300-F / PEW300-...
Embodiment 3
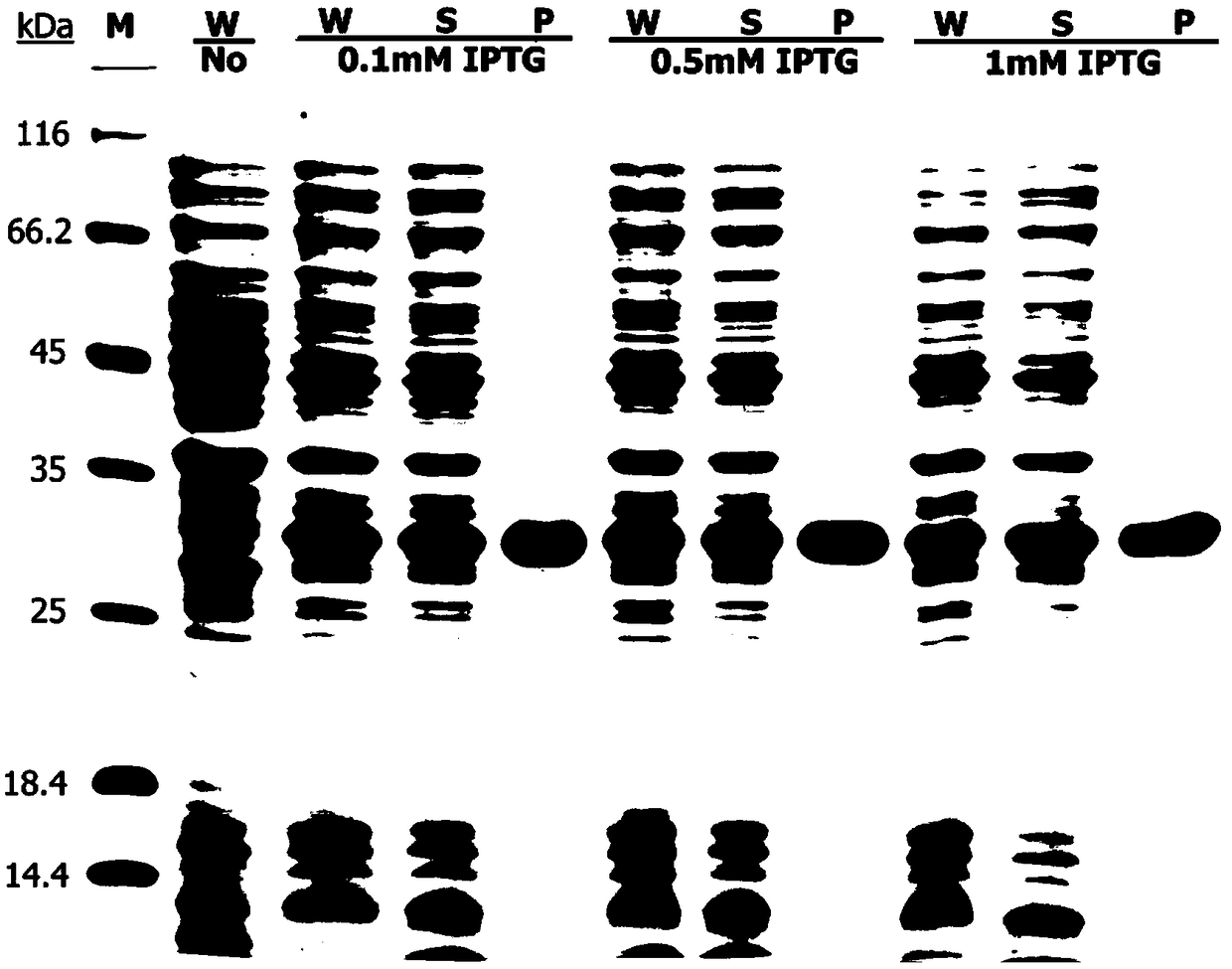
[0066] Example 3: Construction of engineering bacteria expressing PEW300-ELK16 fusion protein in Escherichia coli
[0067] The recombinant expression vector pET-PEW300 constructed in Example 1 was transformed into Escherichia coli BL21 (DE3) competent cells by chemical transformation, and then the bacterial solution was applied to the LB solid plate containing 50ug / ml kanamycin Incubate overnight at 37°C. Among them, BL21 (DE3) competent cells were purchased from Zhenzhi Biotechnology Co., Ltd. The BL21(DE3) engineering bacteria containing the recombinant plasmid pET-PEW300 were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com