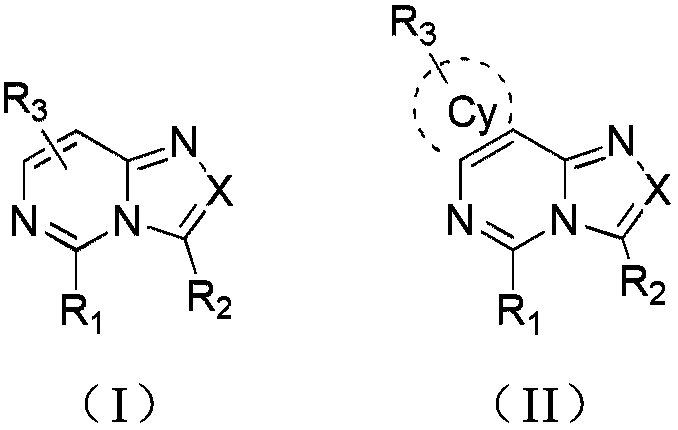
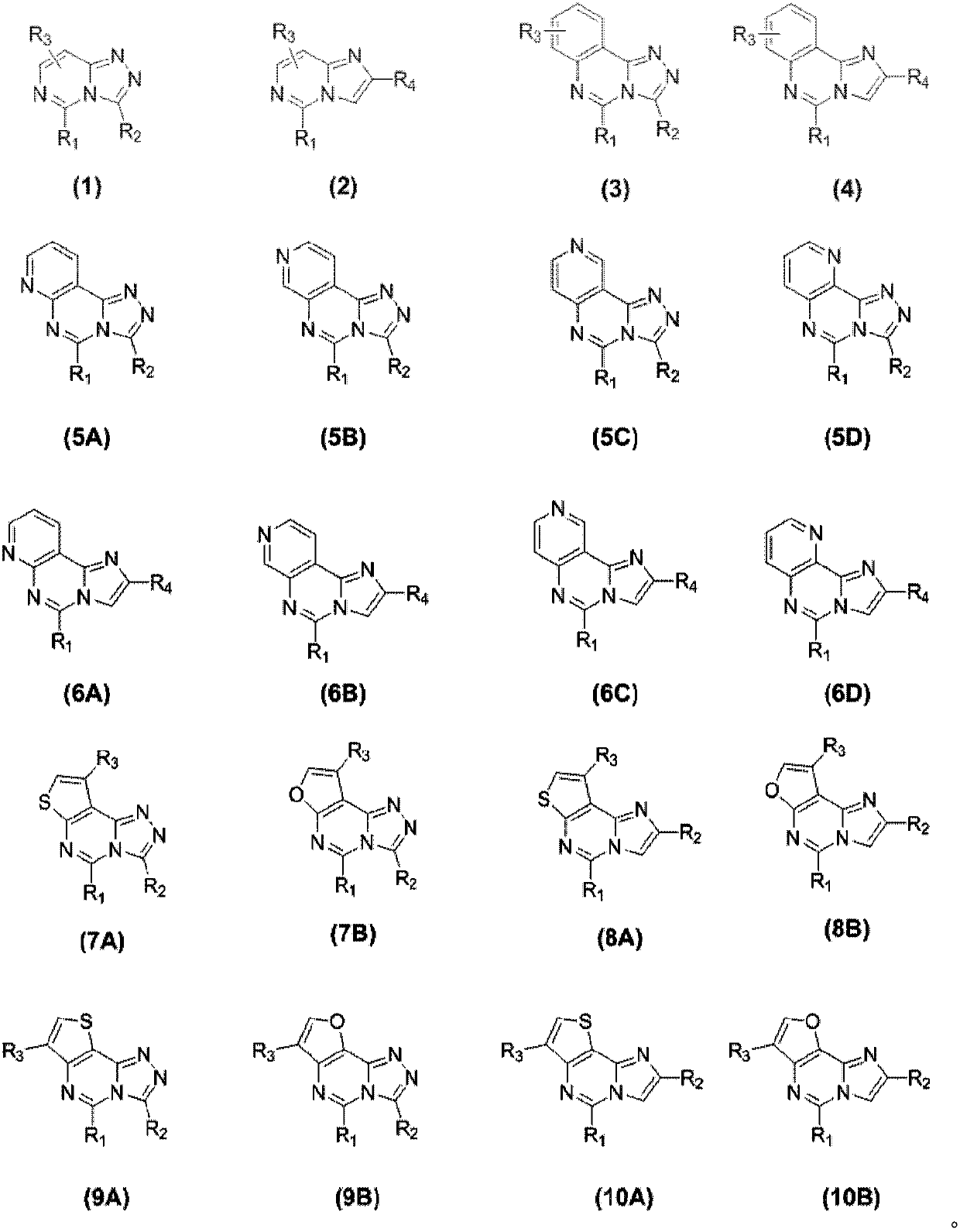
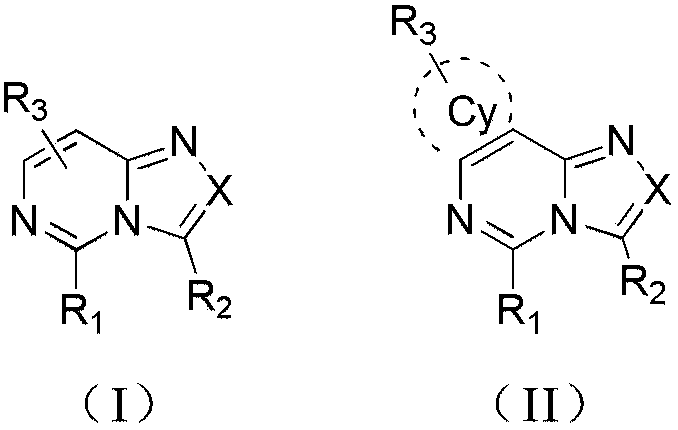
Compound and application thereof in organic electroluminescence field
A technology for electroluminescent devices and compounds, which is applied in the fields of light-emitting materials, organic chemistry, circuits, etc., and can solve the problem of not specifically disclosing organic electroluminescent compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] Preparation of intermediate M1:
[0120]
[0121] Using 2,4-dichloropyrimidine (or its derivatives) as the starting material, first react with hydrazine hydrate to replace the 4-position chlorine atom with relatively high activity of 2,4-dichloropyrimidine (or its derivatives) to generate Intermediate A. Intermediate A further condenses with aldehyde to remove a molecule of water to generate intermediate B. Intermediate B reacts with iodobenzene acetate for oxidative ring closure to generate the first type of intermediate M1.
[0122] Preparation of intermediate M2:
[0123]
[0124] Using 2,4-dichloropyrimidine (or its derivatives) as the starting material, first react with ammonia water to replace the highly active 4-position chlorine atom of 2,4-dichloropyrimidine (or its derivatives) to generate intermediate C . Intermediate C is further reacted with α-bromo(hetero)aryl or alkyl ethyl ketone to generate the second type of intermediate M2.
[0125] The syn...
Embodiment 1
[0126] Embodiment 1: the preparation of compound 1I-12
[0127]
[0128] Preparation of compound 1-1
[0129] After dissolving 2,4-dichloropyrimidine (500g, 3.38mol) in 10L ethanol in a flask, hydrazine hydrate (634g, 10.14mol, 80% aqueous solution) was added dropwise at 5°C under stirring, and the temperature was kept below 10°C. After the dropwise addition, the mixture was naturally raised to room temperature for 1 hour, and the precipitated solid was filtered with suction, washed with water and ethanol, and dried in air to obtain compound 1-1 (389 g, 80%) as an off-white solid.
[0130] Preparation of compound 1-2
[0131] Compound 1-1 (144g, 1mol) was added to a flask containing 1.5L of ethanol, and benzaldehyde (138g, 1.3mol) was added dropwise under stirring at room temperature. After the addition was complete, stirring was continued for 30 minutes, and the obtained solid was filtered and washed with ethanol and Rinse with n-hexane and dry to obtain compound 1-2 (1...
Embodiment 2
[0138] Embodiment 2: the preparation of compound 1II-12
[0139]
[0140] Preparation of compound 2-1
[0141] Add p-chlorophenylboronic acid (7.8g, 50mmol), compound 1-3 (11.5g, 50mmol), potassium carbonate (20.7g, 150mmol) into a flask containing 1,4-dioxane / water (300mL / 100mL) , Pd(PPh 3 ) 4 (578 mg, 0.5 mmol). After the addition was completed, the mixture was reacted at 80° C. for 8 hours under stirring, and the end point of the reaction was monitored by TLC. Separate the liquids, extract the aqueous phase with dichloromethane, combine the organic phases, dry over anhydrous sodium sulfate, filter, and spin off the solvent under reduced pressure. Compound 2-1 (10.7 g, yield 70%) was obtained by separation and purification by column chromatography.
[0142] Preparation of compound 1II-12
[0143] Compound 2-1 (6.12g, 20mmol), compound 1-4 (8.46g, 20mmol), sodium tert-butoxide (5.8g, 60mmol) were added to a flask containing 200mL xylene, and Pd was added under nitrog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com