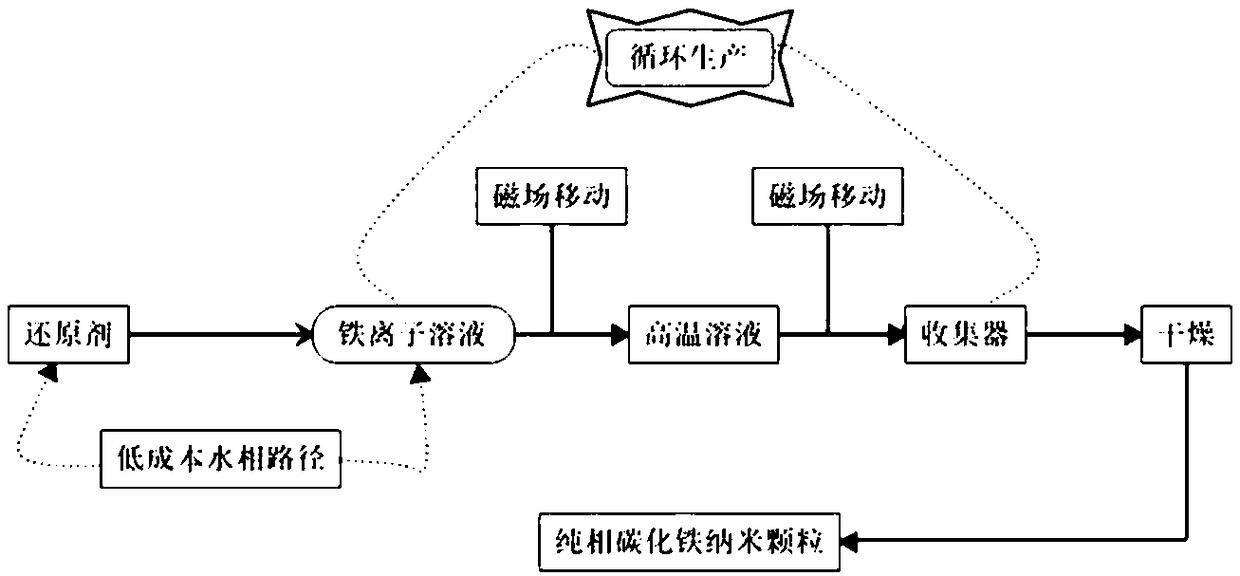
Green continuous pure-phase Fe5C2 nanoparticle preparation method
A nanoparticle and iron carbide technology, applied in chemical instruments and methods, carbon compounds, nanotechnology, etc., can solve problems such as environmental threats and expensive raw materials, and achieve continuity, simple process, and important practical value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Ferrous sulfate is formulated into a 1mol / L solution; 2mol / L sodium borohydride solution is added to the ferrous sulfate solution to produce a black precipitate; then the black precipitate is transferred to the oleylamine solution with a magnetic field, and the molar amount of oleylamine is iron 10 times that of the source; the temperature of oleylamine is 260°C, and after 10 minutes of reaction, the black precipitate is transferred to the receiver by a magnetic field, and then dried at 50°C to obtain a pure-phase iron carbide (Fe 5 C 2 ) nanoparticles.
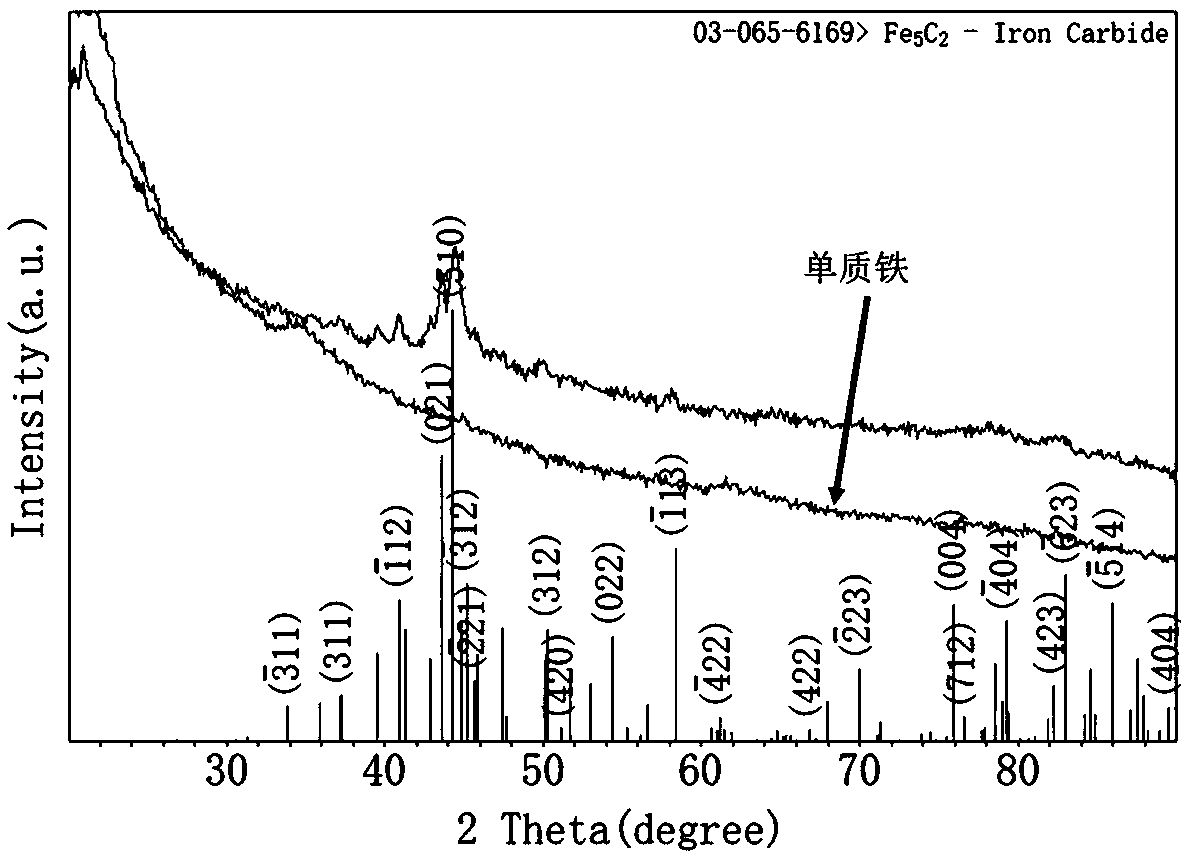
[0055] Such as figure 2 As shown, the obtained intermediate product elemental iron and the final product powder are scanned by X-ray diffractometer between 10 and 80 degrees. For elemental iron, there is no diffraction peak in X-ray diffraction between 10 and 80 degrees. It is proved that the obtained intermediate product is an amorphous elemental iron. For X-ray diffraction of the final product, all diffraction pe...
Embodiment 2
[0057] Ferric sulfate is formulated into a 1mol / L solution; 3mol / L sodium borohydride solution is added to the ferric sulfate solution to produce a black precipitate; then the black precipitate is transferred to the cetylamine solution with a magnetic field, and the molar amount of cetylamine is iron 20 times that of the source; the temperature of hexadecylamine is 270°C, and after 30 minutes of reaction, the black precipitate is transferred to the receiver by a magnetic field, and then dried at 120°C to obtain pure phase iron carbide (Fe 5 C 2 ) nanoparticles.
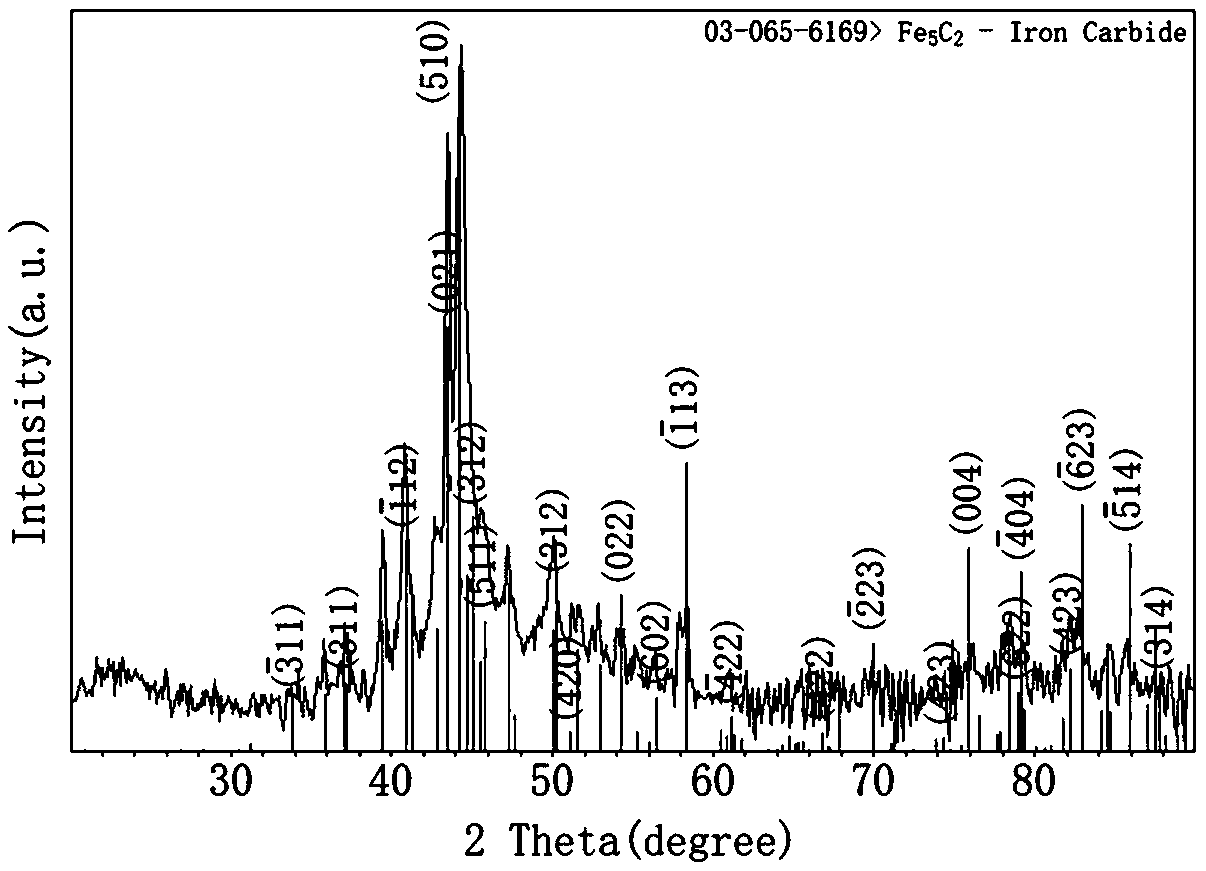
[0058] image 3 Give all the diffraction peaks and Fe of the obtained powder using the X-ray diffraction pattern 5 C 2 The standard card (PDF: 03-065-6169) is completely consistent without any miscellaneous peaks, which proves that the obtained powder is Fe 5 C 2 pure phase. Among them, the standard card (PDF: 03-065-6169) has a monoclinic phase structure, and its structure is determined to be monoclinic C2 / c. ...
Embodiment 3
[0060] Ferric nitrate is mixed with 1mol / L solution; 3mol / L sodium borohydride solution is added in ferrous nitrate solution, produces black precipitate; Then black precipitate is transferred in the octadecylamine solution with magnetic field, and the molar weight of octadecylamine is 40 times that of the iron source; the temperature of octadecylamine is 300°C, and after 60 minutes of reaction, the black precipitate is transferred to the receiver by a magnetic field, and then dried at 200°C to obtain pure phase iron carbide (Fe 5 C 2 ) nanoparticles.
[0061] Figure 4 Given the transmission electron diffraction pattern of the obtained powder, it can be seen from the figure that Fe 5 C 2 The nanoparticles are basically in the shape of regular spheres. Each ball is formed by agglomerating small balls with very small particles. The particle size of the nanospheres is about less than 20 nanometers, which is basically consistent with the grain size obtained by calculating the h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grain size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Coercivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com