A purification process suitable for treating lithium-containing minerals by sodium salt method
A purification process, sodium salt technology, which is applied in photography technology, photography auxiliary technology, instruments, etc., can solve the problems of low utilization value of waste residue, complicated process flow, high cost of impurity removal, etc., achieve low cell voltage, shorten process flow, reduce The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
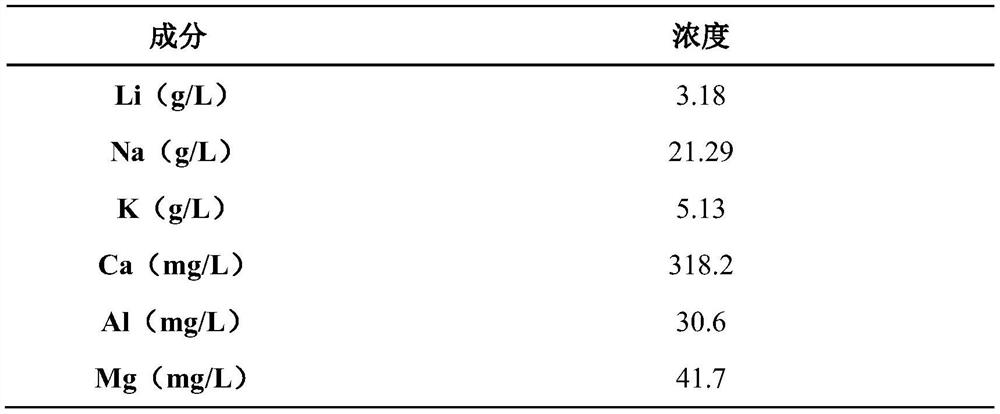
[0038] 1) The sodium superionic conductor type NaV in the sodium-deficient state 2 (PO 4 ) 3 , with acetylene black and polyvinylidene fluoride (PVDF) at a mass ratio of 90:5:5, uniformly dispersed in N-methylpyrrolidone (NMP) solvent, then evenly coated on both sides of the aluminum plate, and dried at 120 ° C 12h, as the cathode plate; nickel foam was used as the anode, and the leaching solution of lepidolite sodium sulfate sintering method was used as the electrolyte solution. Under the current density of electrolysis for 15h, the adsorption of Li + 、Na + .
[0039] Table 1
[0040]
[0041] 2) (Li, Na) loaded with Li and Na obtained in step 1) 3 V 2 (PO 4 ) 3 The cathode plate is used as the anode, the nickel foam is used as the cathode, and the 1mol / L sodium sulfate solution is used as the electrolyte. The electrolytic cell is divided into an anode chamber and a cathode chamber by an anion semipermeable membrane. Analyze at a current density of 0.1A / g for 15 ...
Embodiment 2
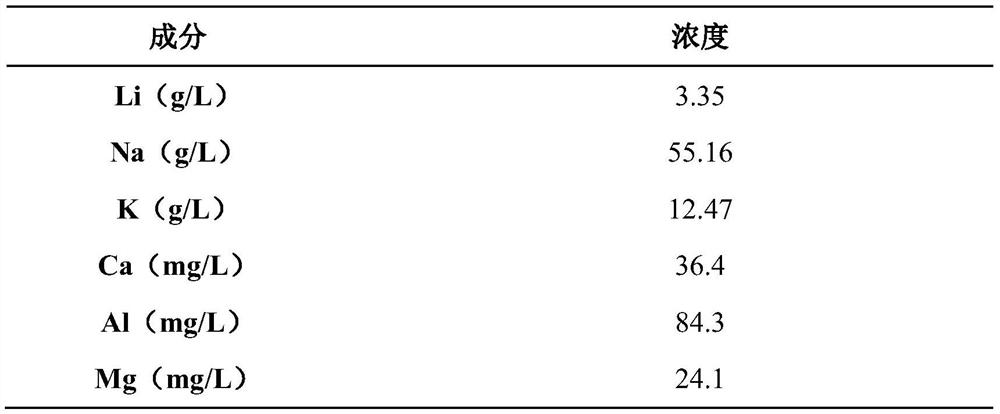
[0044] 1) The sodium-deficient Prussian blue analog FeFe(CN) 6 , and high-purity graphite, polyvinylidene fluoride (PVDF) in a mass ratio of 90:5:5, uniformly dispersed in N-methylpyrrolidone (NMP) solvent, and then evenly coated on both sides of the stainless steel plate, at 120 ° C Dry for 12 hours, and use it as the cathode plate; use graphite as the anode, and the leaching solution obtained by the lepidolite sodium chloride roasting method is used as the electrolyte. Electrolysis at a current density of g for 8h, adsorption of Li + 、Na + .
[0045] Table 2
[0046]
[0047] 2) (Li, Na) loaded with Li and Na obtained in step 1) 2 FeFe(CN) 6 The cathode plate is used as the anode, the graphite is used as the cathode, and 0.3mol / L sodium hydroxide solution is used as the electrolyte. The electrolytic cell is divided into an anode chamber and a cathode chamber by an anion semipermeable membrane. At 20°C, a cell voltage of 1.0V is applied. Analyze at a current density ...
Embodiment 3
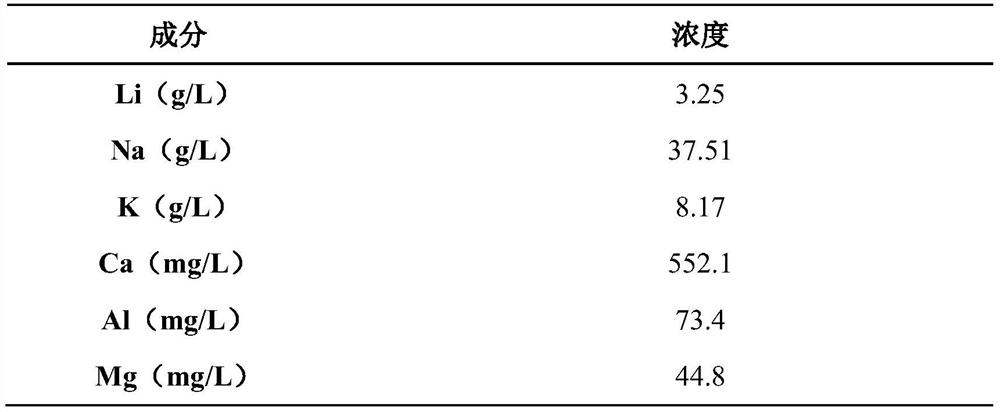
[0050] 1) The tunnel-type Na in the sodium-deficient state 0.22 MnO 2 , with high-purity graphite and polyvinylidene fluoride (PVDF) at a mass ratio of 8:1:1, uniformly dispersed in N-methylpyrrolidone (NMP) solvent, and then evenly coated on both sides of the aluminum plate, baked at 80 ° C Dry for 12 hours, as the cathode plate; use nickel foam as the anode, and the leaching solution of spodumene sodium sulfate pressure cooking method as the electrolyte. Electrolyzed for 12h at a current density of / g, the adsorption of Li + , Na + .
[0051] table 3
[0052]
[0053] 2) (Li, Na) loaded with Li and Na obtained in step 1) 0.66 MnO 2 Cathode plate as anode, nickel foam as cathode, 2mol / L Na 2 SO 4 +1mol / L NaOH solution is used as the electrolyte, and the electrolytic cell is divided into an anode chamber and a cathode chamber by an anion semipermeable membrane. Desorption releases Li and Na ions from the plate loaded with Li and Na into the anolyte, and the desorbe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com