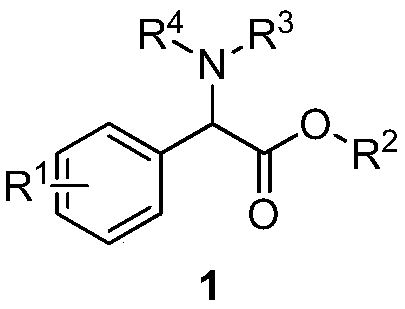
Application of surface-modified sludge carbon catalyst to carbenoid insertion N-H reaction
A technology of amine compounds and methyl phenylacetate, which is applied in the application field of surface-modified sludge carbon catalysts in the carbene intercalation N-H reaction, to achieve the effects of easy separation and purification, low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Sludge charcoal catalyst preparation: (1) Take a small amount of sludge from municipal domestic sewage plants and dry it at 110°C until the weight remains unchanged; (2) Heat the sludge obtained in (1) in a nitrogen atmosphere to 5°C The heating rate per minute was raised to 600°C, and the calcination time was 6h. After being cooled to 25°C, and mechanically pulverized, a sludge charcoal carrier with high specific surface area and high porosity was obtained. Take 5L of the sludge charcoal obtained in (2), soak it with 5L of perchloric acid with a mass fraction of 30wt.% for 24h, then rinse the soaked sludge charcoal with deionized water until the pH value of the effluent is greater than 6, and then treat the The final sludge charcoal was air-dried at room temperature to obtain a perchloric acid surface-modified sludge charcoal catalyst.
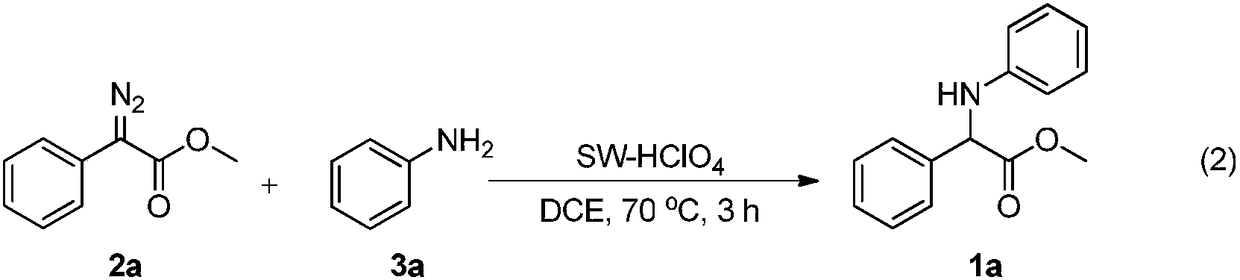
[0031] At room temperature, successively weigh phenyldiazoacetate methyl ester 2a (0.5mmol), aniline 3a (0.65mmol), SW-HC...
Embodiment 2
[0033]
[0034] At room temperature, successively weigh phenyldiazoacetate methyl ester 2a (0.5mmol), p-trifluoromethylaniline 3b (0.65mmol), SW-HClO 4 (100 mg) was added to a 25 mL Schlenk reaction flask under air atmosphere, 5 mL of 1,2-dichloroethane was added, stirred at room temperature for 2 minutes, and reacted in an oil bath at 40° C. for 1 hour. After the reaction, the mixture was cooled to room temperature, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=10:1 ) to obtain the target product 1b (122 mg, yield 79%) as a white solid. The target product was confirmed by NMR and high-resolution mass spectrometry. The sludge charcoal catalyst used in this example is consistent with Example 1.
Embodiment 3
[0036]
[0037] At room temperature, successively weigh phenyldiazoacetate methyl ester 2a (0.5mmol), m-trifluoromethylaniline 3c (0.65mmol), SW-HClO 4 (100 mg) was added to a 25 mL Schlenk reaction flask under air atmosphere, 5 mL of 1,2-dichloroethane was added, stirred at room temperature for 2 minutes, and reacted in an oil bath at 50° C. for 2 hours. After the reaction, the mixture was cooled to room temperature, and the volatile components were removed under reduced pressure, and then separated by silica gel column chromatography (eluent was petroleum ether (60-90°C) / ethyl acetate, v / v=10:1 ), to obtain the target product 1c (100 mg, yield 65%) as a white solid. The target product was confirmed by NMR and high-resolution mass spectrometry. The sludge charcoal catalyst used in this example is consistent with Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com