Aldehyde-ketone reductase mutant and its application
A reductase and mutant technology, applied to aldehyde-ketone reductase mutants and their application fields, can solve the problems of low asymmetric reduction activity and low substrate concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Construction and screening of aldehyde and ketone reductase mutant library
[0026] The aldehyde and ketone reductase gene (shown in the amino acid sequence of SEQ ID No.2, and the nucleotide sequence shown in SEQ ID No.1) was constructed as an expression vector pET-28b(+)-klakr, transformed into Escherichia coli, and obtained the starting strain E .coliBL21(DE3) / pET28b-pklakr.
[0027] The preparation of the aldehyde and ketone reductase mutant library was achieved through 4 rounds of site-directed saturation mutagenesis. The primers were designed as shown in Table 1. In the first round, the vector pET-28b(+)-klakr was used as a template, and I125F and I125R in Table 1 were used as primers , through saturation mutation PCR, the 125th isoleucine in the amino acid sequence of aldehyde and ketone reductase shown in SEQ ID No.2 was mutated into the remaining 19 kinds of amino acids, and transformed, plated, and the aldehyde and ketone reductase mutation was obta...
Embodiment 2
[0031] Example 2: Induced expression of aldehyde and ketone reductase parent, mutant and glucose dehydrogenase
[0032] Glucose dehydrogenase gene esgdh (nucleotide sequence shown in SEQ ID No.3, amino acid sequence shown in SEQ ID No.4) was cloned from Exiguobacterium sibiricum, and connected to pET-28b(+) by double enzyme digestion On the vector, the recombinant plasmid was introduced into Escherichia coli E.coli BL21(DE3) to obtain the recombinant glucose dehydrogenase strain E.coliBL21(DE3) / pET28b-esgdh.
[0033] The starting strain E.coli BL21(DE3) / pET28b-pklakr and the aldehyde and ketone reductase mutant strain of Example 1 and the recombinant glucose dehydrogenase strain E.coli BL21(DE3) / pET28b-esgdh were respectively inoculated to a final concentration of 50 μg / In LB liquid medium containing 50 μg / mL kanamycin, cultured at 37°C for 9 hours, inoculated into fresh LB liquid medium containing 50 μg / mL kanamycin at a volume fraction of 2% (v / v), 37 Cultivate at 180 rpm ...
Embodiment 3
[0034] Example 3: Mutant library screening
[0035] Mix the wet cells of the mutant strain induced and expressed in Example 2 and the wet cells of glucose dehydrogenase at a mass ratio of 3:1, and add the amount of 50 g / L of the total amount of cells into pH 7.5, 100 mM phosphate buffer for resuspension , ultrasonic crushing on ice-water mixture for 10 minutes, ultrasonic crushing conditions: power of 400W, crushing for 1 second, pausing for 1 second, to obtain the crude enzyme solution of the mutant strain. Under the same conditions, replace the wet cells of the mutant strain with the starting strain E.coli BL21(DE3) / pET28b-pklakr to prepare the crude enzyme solution of the starting strain.
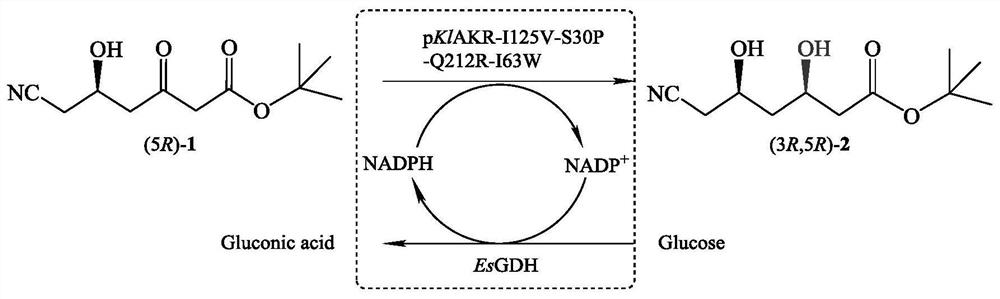
[0036] The crude enzyme solution of the mutant strain or the crude enzyme solution of the original strain was used as a catalyst, tert-butyl 6-cyano-(5R)-hydroxy-3-oxohexanoate was used as a substrate, glucose was used as an auxiliary substrate, and no exogenous sources were added. NADP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
| catalytic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com