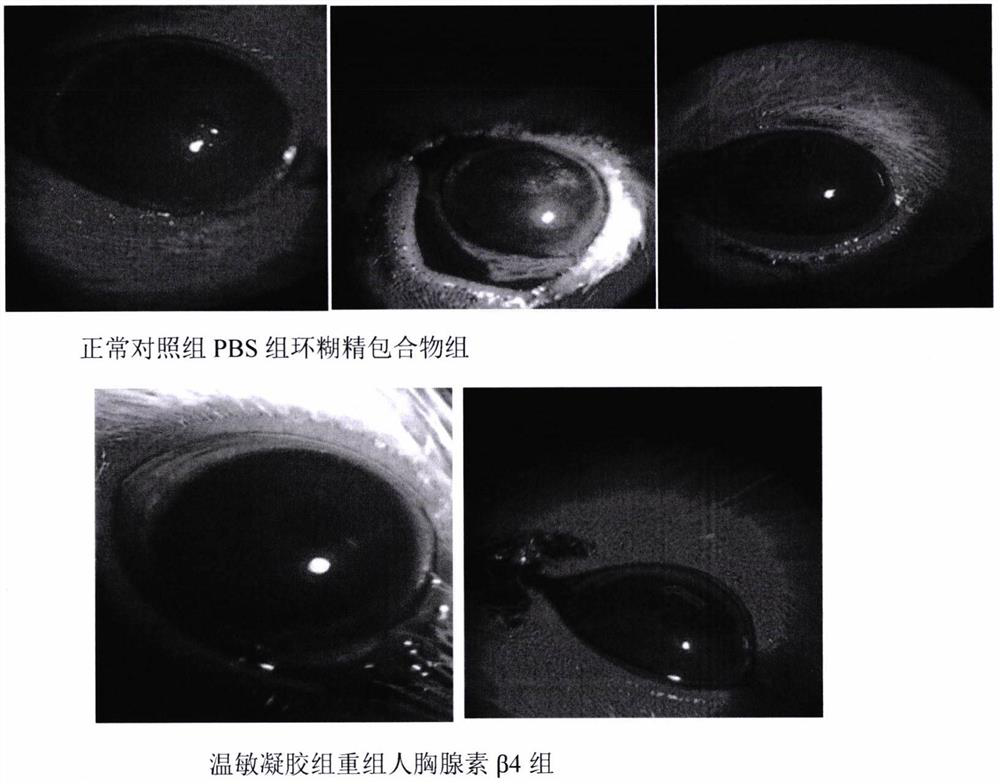
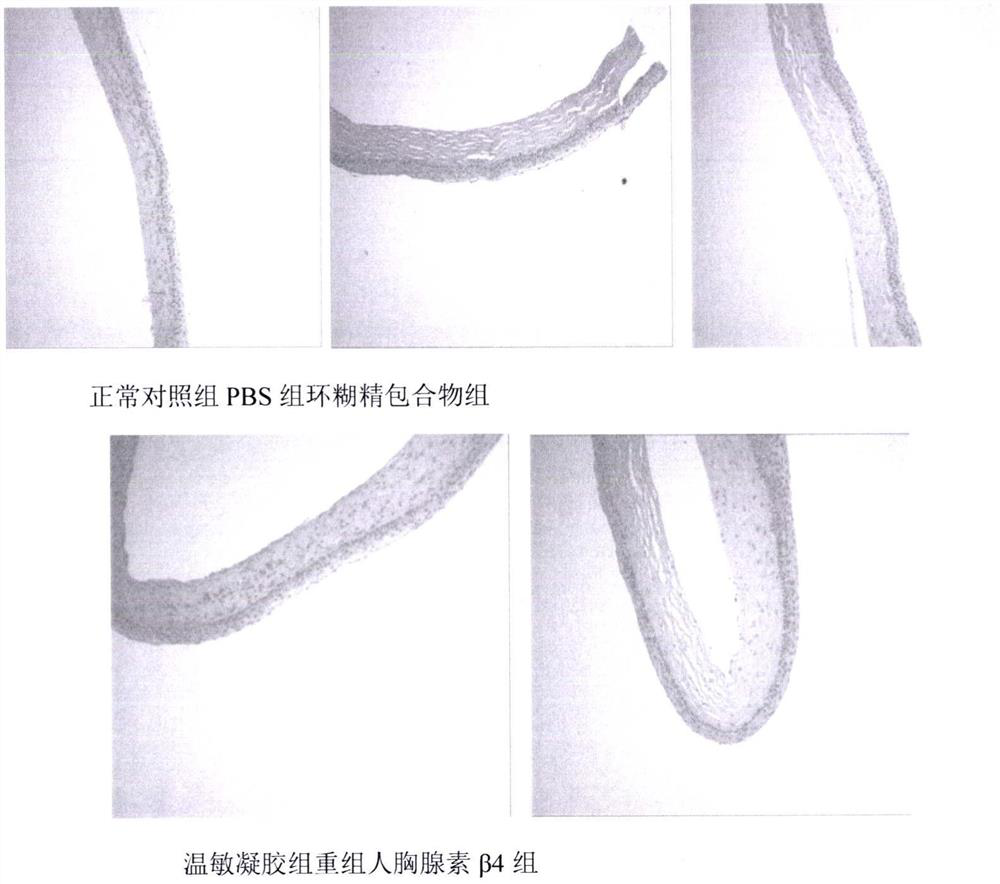
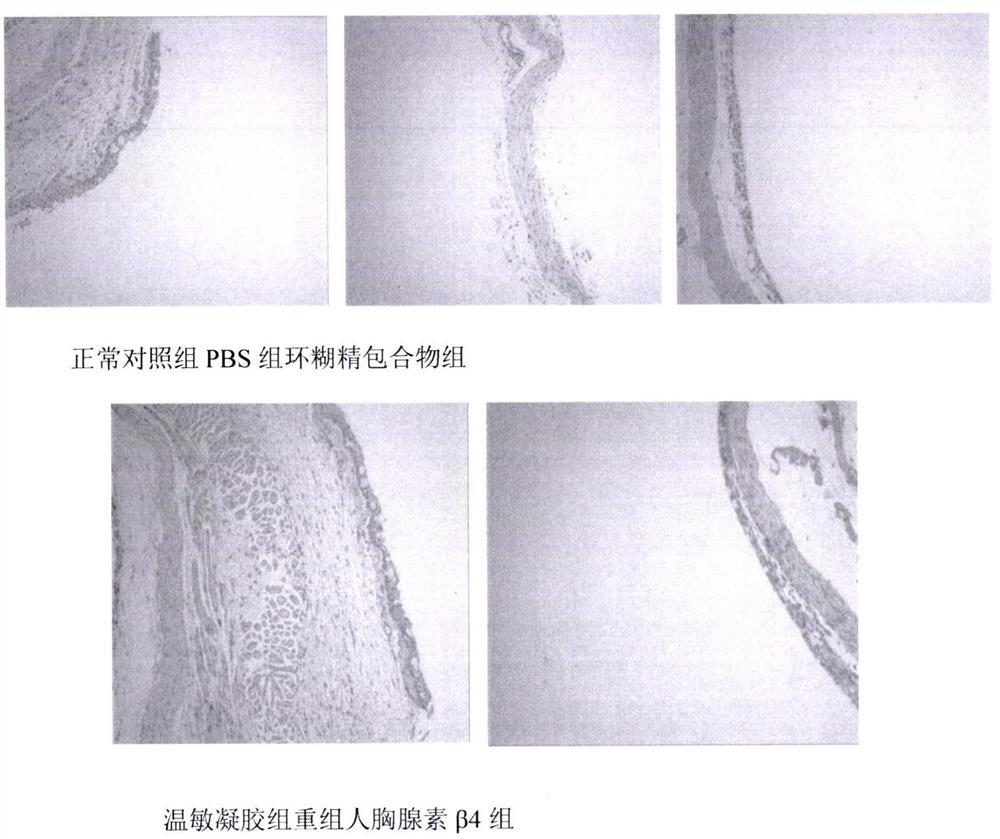
A temperature-sensitive recombinant human thymosin β4 ophthalmic in situ gel preparation
A temperature-sensitive, in-situ gel technology, which can be used in medical preparations without active ingredients, medical preparations containing active ingredients, and drug combinations, which can solve problems such as side effects, discomfort, and short residence time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Packing and comparison of different molar ratios (1:10, 1:20, 1:40) of recombinant human thymosin β4 and hydroxypropyl-β-cyclodextrin
[0028] Methods: Recombinant human thymosin β4 cyclodextrin, hydroxypropyl-β-cyclodextrin and recombinant human thymosin β4 prepared according to different ratios were measured by FT-IR and potassium bromide tablet method respectively. IR of fine clathrate and hydroxypropyl-β-cyclodextrin. Weigh 0.3mg~2mg respectively, add potassium bromide as a carrier, grind evenly with a mortar, press into tablets, put it into a Fourier transform infrared spectrometer, the spectral scanning range is 4000~400cm-1, and measure each group infrared absorption spectrum. According to the change of absorption peak of each substance in the scanning range, the formation of clathrate can be judged.
[0029] Weigh the recombinant human thymosin β4 freeze-dried product, hydroxypropyl-β-cyclodextrin, recombinant human thymosin β4-cyclodextrin inclusio...
Embodiment 2
[0031] Example 2: Effects of different ratios of P407 and P188 on the temperature of thermosensitive gel
[0032] Beroxamer 407 is the most studied temperature-sensitive polymer, and it is the most frequently used polymer excipient in the preparation of temperature-sensitive gels. The 20% to 30% solution of Beroxamer 407 has the property of reverse gelling. Beroxamer 188 will not cause gelation when used alone in the concentration range of 10% to 30%, so the two are usually used in combination. By investigating the relationship between the concentration of poloxamer 407 and boloxamer 188 and the gelation temperature, the experimental results show that as the concentration of P407 increases, the gelation temperature decreases. As the temperature increases, the gelation temperature also gradually increases, as shown in Table 1-1.
[0033] Table 1-1 The influence of the composition concentration of poloxamer on the gelling temperature
[0034]
[0035]
[0036] By compari...
Embodiment 3
[0037] Embodiment three: stability comparison
[0038] The degradation rates of recombinant human thymosin β4 aqueous solution, recombinant human thymosin β4 cyclodextrin inclusion complex aqueous solution prepared in different proportions and recombinant human thymosin β4 cyclodextrin inclusion complex thermosensitive gel solution were compared by content determination.
[0039] Results: Through the experimental investigation on the stability of drugs and preparations, the thermosensitive gel of recombinant human thymosin β4 cyclodextrin inclusion compound has good stability within one month, which is more stable than recombinant human thymosin β4, and the degradation rate is lower than that of recombinant human thymosin. Prime β4 is slow. Comparing the preparations with three ratios, the preparation prepared when the ratio is 1:10 is the most stable.
[0040] 0 1 5 10 30 N1:10 100% 97.6% 95.7% 90.4% 89.6% N1:20 100% 98.2% 96.5% 89% 68.4% ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com