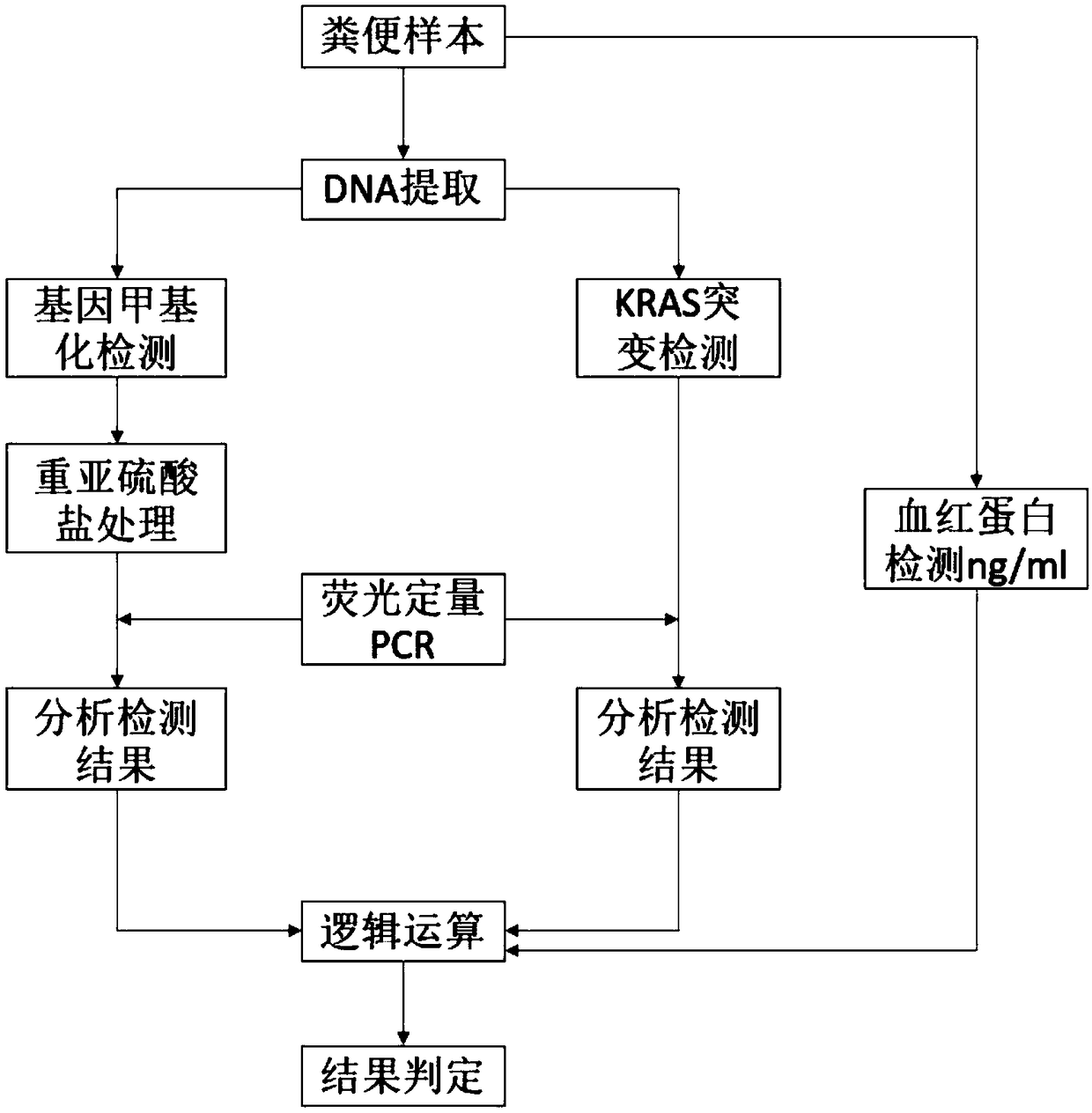
Kit for detecting colorectal cancer and precancerous lesions and use method thereof
A technology for precancerous lesions and colorectal cancer, applied in the field of biotechnology and diagnostic research, can solve the problem of low screening specificity, low screening sensitivity and specificity of guaiac fecal occult blood test, and inability to detect intestinal distant End lesions and other problems, to achieve the effect of reducing the rate of missed diagnosis, high accuracy, and convenient sampling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Stool sample collection and DNA extraction
[0043] 1. Stool sample collection and processing:
[0044] Immediately after the person excretes feces, collect 5g of feces samples and put them into the collection tube, which contains 15ml of feces preservation solution. Shake the collection tube fully to fully mix the feces samples and the feces preservation solution;
[0045] Reagent name
15ml system / part
0.5M EDTA, pH8.0, sterile
3ml
5M NaCl solution, sterile
30ul
1M Tris buffer, pH7.6
7.5ml
4.47ml
[0046] 2. Extraction of stool sample DNA:
[0047] (1) cell pellet
[0048] Draw 1ml of stool sample liquid into a centrifuge tube, pipette and mix well, and then centrifuge at 10,000rpm for 3 minutes to precipitate intestinal exfoliated cells to the bottom of the tube, retain the precipitate, and remove the supernatant;
[0049] (2) Cell cleavage releases DNA
[0050] Add 800ul of feces ...
Embodiment 2
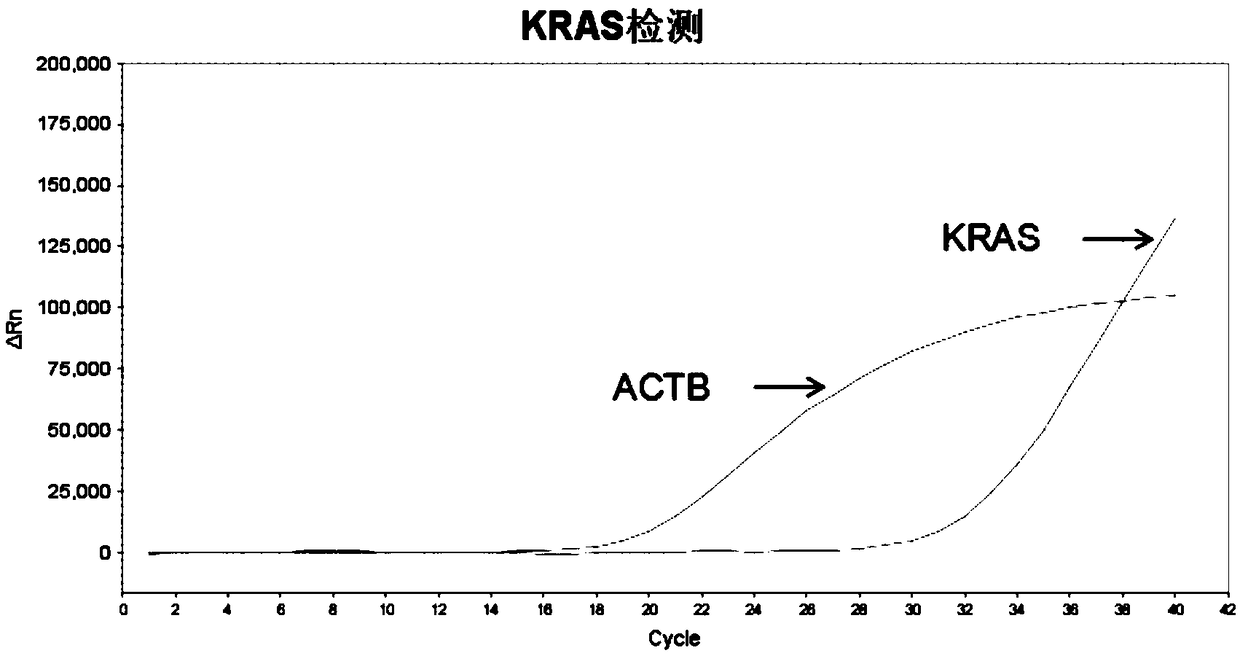
[0064] Example 2: KRAS mutation detection in stool sample DNA
[0065] 1. Reagent preparation
[0066] (1) Take out the internal reference gene (ACTB) reaction solution and KRAS reaction solution from the refrigerator, equilibrate to room temperature, fully dissolve, and centrifuge quickly for 10 seconds; take out the PCR reaction premix reagent (master mix) from the refrigerator at 4°C, and spin for 10 seconds . The PCR premix reagent is a commercial quantitative PCR reaction mixed reagent; the reaction solution consists of probes (synthesized by a professional probe synthesis company), primers (synthesized by a professional primer synthesis company), water, KRAS and internal reference gene (ACTB) each There is a reaction solution. The probe and primer sequences are from the literature "Optimized Multiplex Detection of 7KRAS Mutations by Taqman Allele-SpecificqPCR". The specific probe and primer sequences are as follows:
[0067] KRAS:
[0068]
[0069] Remarks: Primer...
Embodiment 3
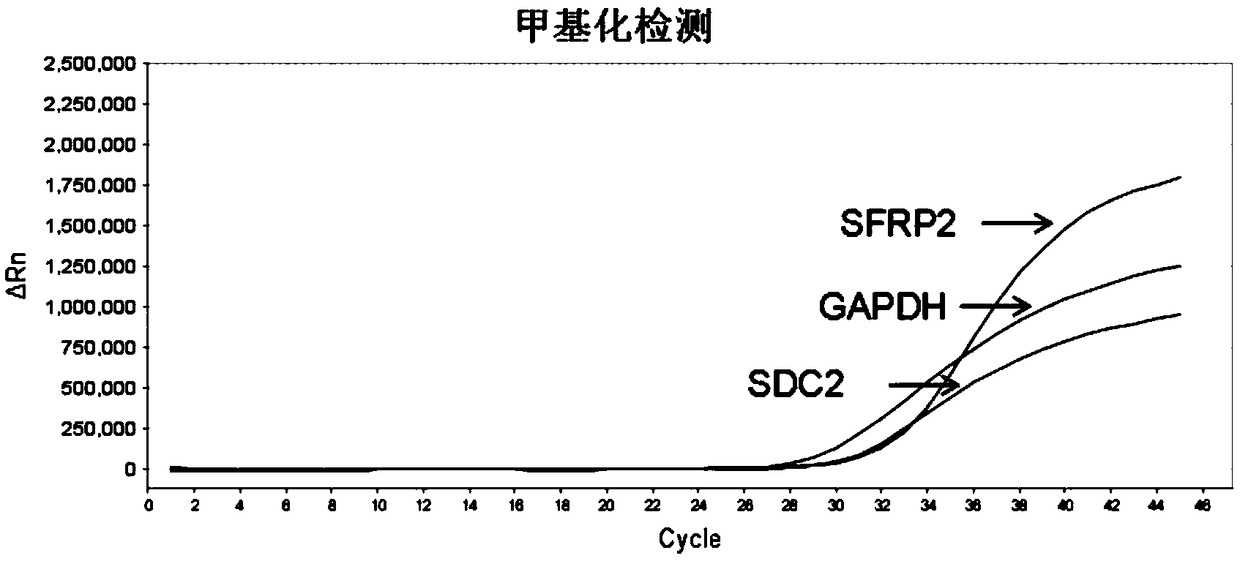
[0083] Example 3: Methylation detection of human DNA in stool samples
[0084] 1. Sulphite conversion
[0085] (1) Add 1 ug of fecal sample DNA to be treated in a 2.0 ml centrifuge tube, that is, the DNA-containing solution obtained in Example 1, the recommended dosage of 10 ng / ul concentration is 100 ul;
[0086] (2) Add 300ul of sulfite conversion solution and 50ul of protection solution to the centrifuge tube in step (1), cover the centrifuge tube tightly, vortex to mix, and centrifuge briefly;
[0087] The sulfite conversion solution is an aqueous solution containing 50% (m / v) sodium bisulfite and 15% (m / v) sodium sulfite, and the pH value is 5.5;
[0088] The protective solution is a mixture of quinol dimethacrylate and diethylene glycol dimethyl ether prepared in a ratio of 200mg: 1.5ml;
[0089] (3) Seal the centrifuge tube in step (2) and place it in a constant temperature shaking incubator, and incubate at 95°C for 20 minutes;
[0090] (4) Immediately take out the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com