A method of separating methane and nitrogen
A technology for separating methane and nitrogen, which is applied in separation methods, chemical instruments and methods, methane capture, etc., can solve the problems of poor hydrothermal stability, high ligand price and high preparation cost, and achieves good water resistance and simple purification steps. , the effect of low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
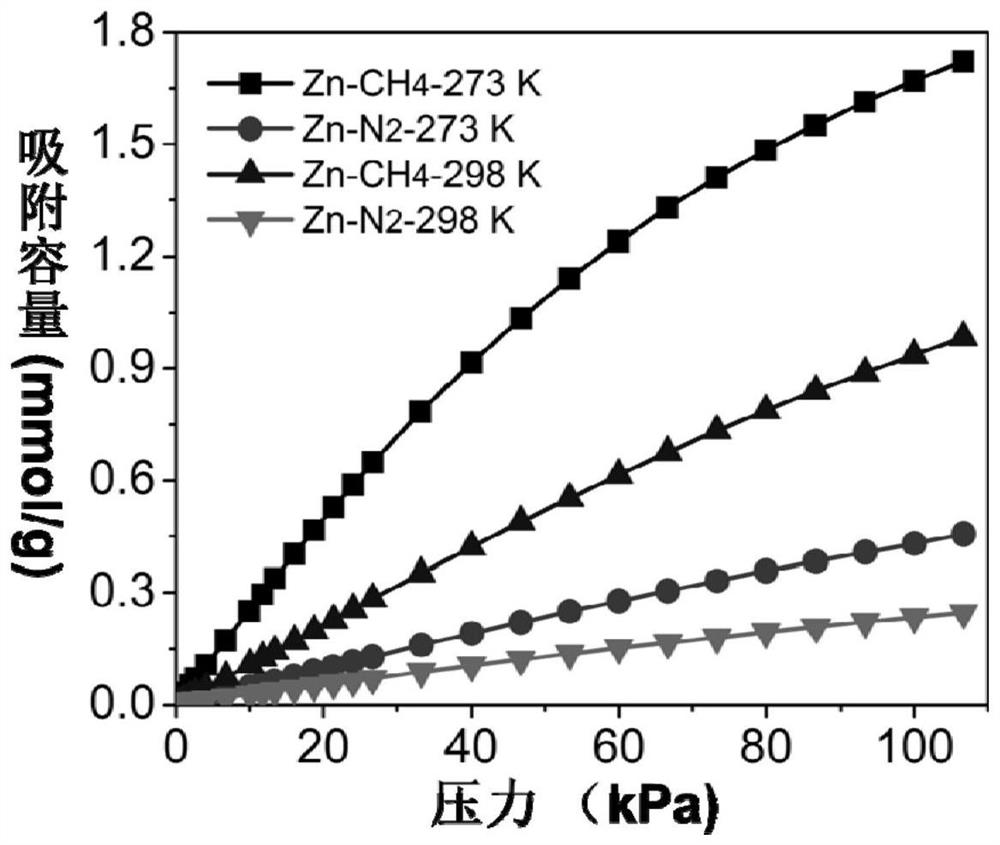
[0046] Mix 1.5mmol of zinc acetate dihydrate, 1.5mmol of 2,5-dihydroxy-1,4-benzoquinone, and 10mL of deionized water, put them into a 25mL hydrothermal reactor, and stir and react at room temperature for 12 hours. After the reaction is completed, the solid obtained from the reaction is centrifuged and washed several times with deionized water and ethanol to obtain a purified metal-organic framework material. The purified adsorbent was vacuum degassed at 100 °C for 12 h to obtain the desolvated adsorbent, followed by gas adsorption.
[0047] To test the adsorption-separation performance of the above-synthesized MOFs, a single-component adsorption isotherm of methane and nitrogen was carried out using the above-mentioned adsorbents. Take an appropriate amount of adsorbent, and the adsorption temperature is 0°C and 25°C. The adsorption isotherm curve is shown in the appendix figure 1 . After testing, at 0°C and 1 bar, the adsorption amount of methane is as high as 1.72mmol / g, ...
Embodiment 2
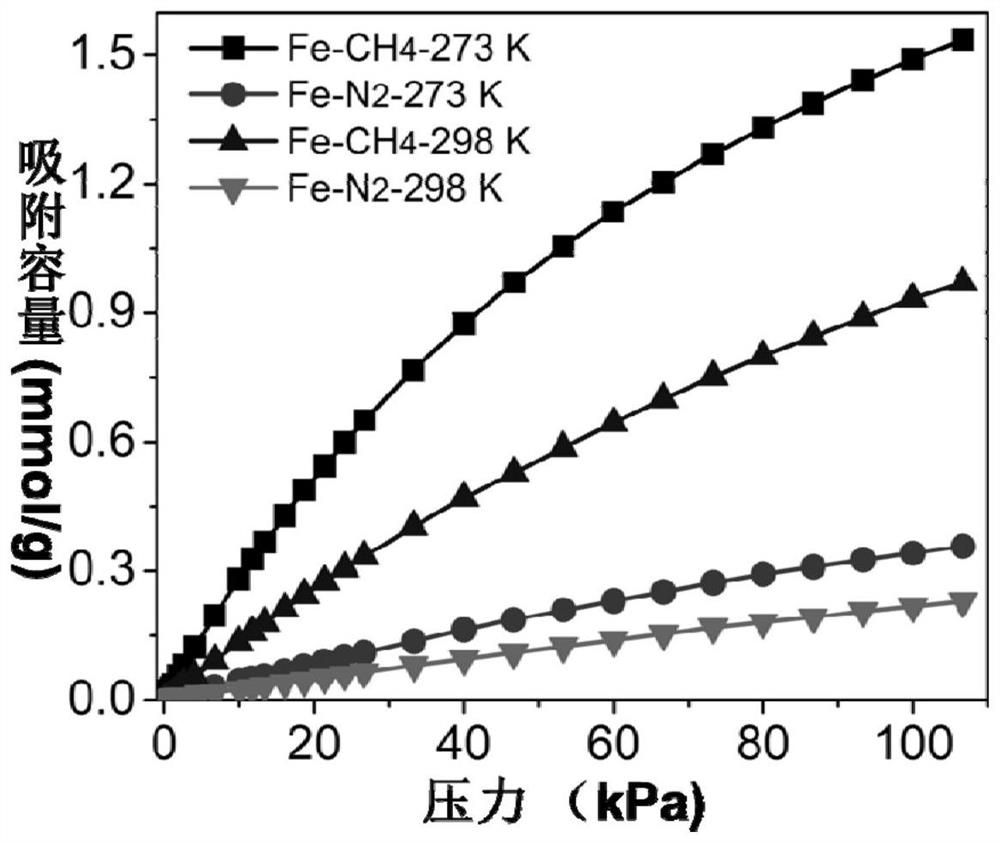
[0050] Mix 1.5mmol ferric chloride hexahydrate, 1.5mmol 2,5-dihydroxy-1,4-benzoquinone, and 10mL deionized water, put them into a 25mL hydrothermal reactor, and stir and react at room temperature for 12 hours. After the reaction is completed, the solid obtained from the reaction is centrifuged and washed several times with deionized water and ethanol to obtain a purified metal-organic framework material. The purified adsorbent was vacuum degassed at 100 °C for 12 h to obtain the desolvated adsorbent, followed by gas adsorption.
[0051] To test the adsorption-separation performance of the above-synthesized MOFs, a single-component adsorption isotherm of methane and nitrogen was carried out using the above-mentioned adsorbents. Take an appropriate amount of adsorbent, and the adsorption temperature is 0°C and 25°C. The adsorption isotherm curve is shown in the appendix figure 2 . After testing, at 0°C and 1bar, the adsorption capacity of methane is as high as 1.54mmol / g, an...
Embodiment 3
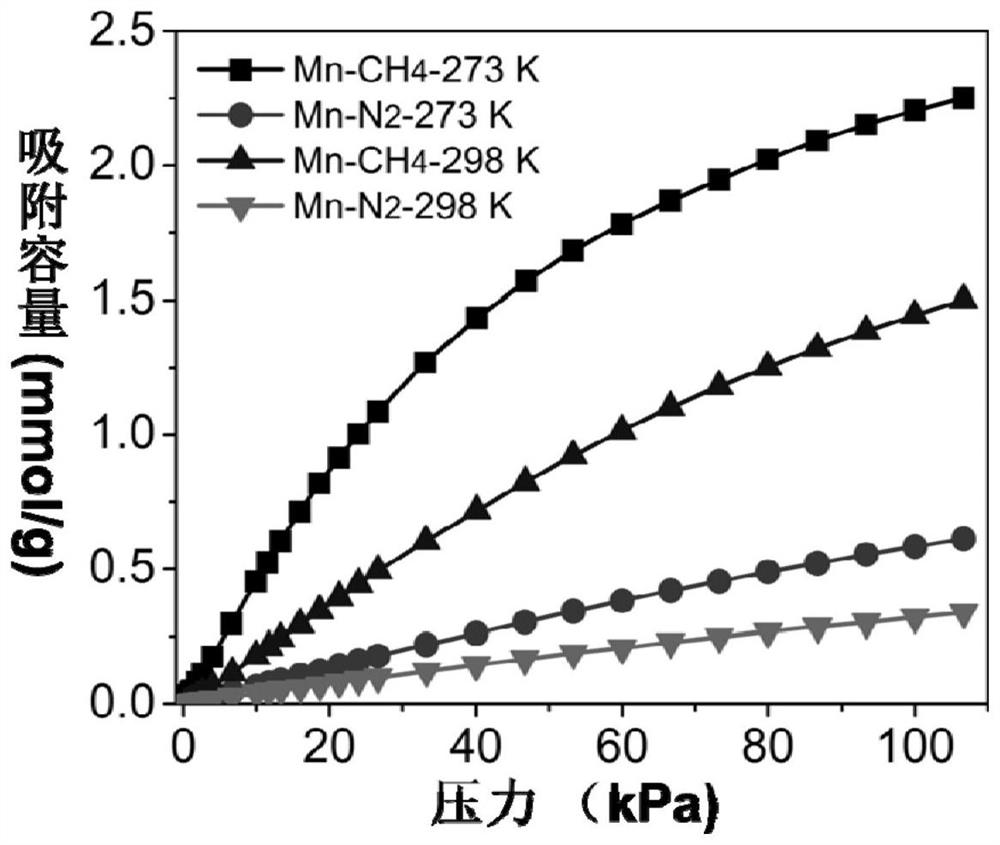
[0054] Mix 2 mmol of anhydrous manganese chloride, 1.5 mmol of 2,5-dihydroxy-1,4-benzoquinone, and 15 mL of deionized water, put them into a 25 mL hydrothermal reactor, and stir and react at room temperature for 12 hours. After the reaction is completed, the solid obtained from the reaction is centrifuged and washed several times with deionized water and ethanol to obtain a purified metal-organic framework material. The purified adsorbent was vacuum degassed at 100 °C for 12 h to obtain the desolvated adsorbent, followed by gas adsorption.
[0055] To test the adsorption-separation performance of the above-synthesized MOFs, a single-component adsorption isotherm of methane and nitrogen was carried out using the above-mentioned adsorbents. The adsorption isotherm curve is shown in the appendix image 3 . Take an appropriate amount of adsorbent, and the adsorption temperature is 0 degrees and 25 degrees. At 0°C and 1 bar, the adsorption amount of methane reaches 2.25mmol / g, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com