P-nitrobenzoic acid uranyl coordination compound photocatalyst and preparation method thereof
A technology of uranyl nitrobenzoate and p-nitrobenzoic acid is applied in the field of uranyl p-nitrobenzoate complex photocatalyst and its preparation, and can solve the problem of narrow light response range, low quantum efficiency, low quantum efficiency, etc. problem, to achieve the effect of avoiding secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
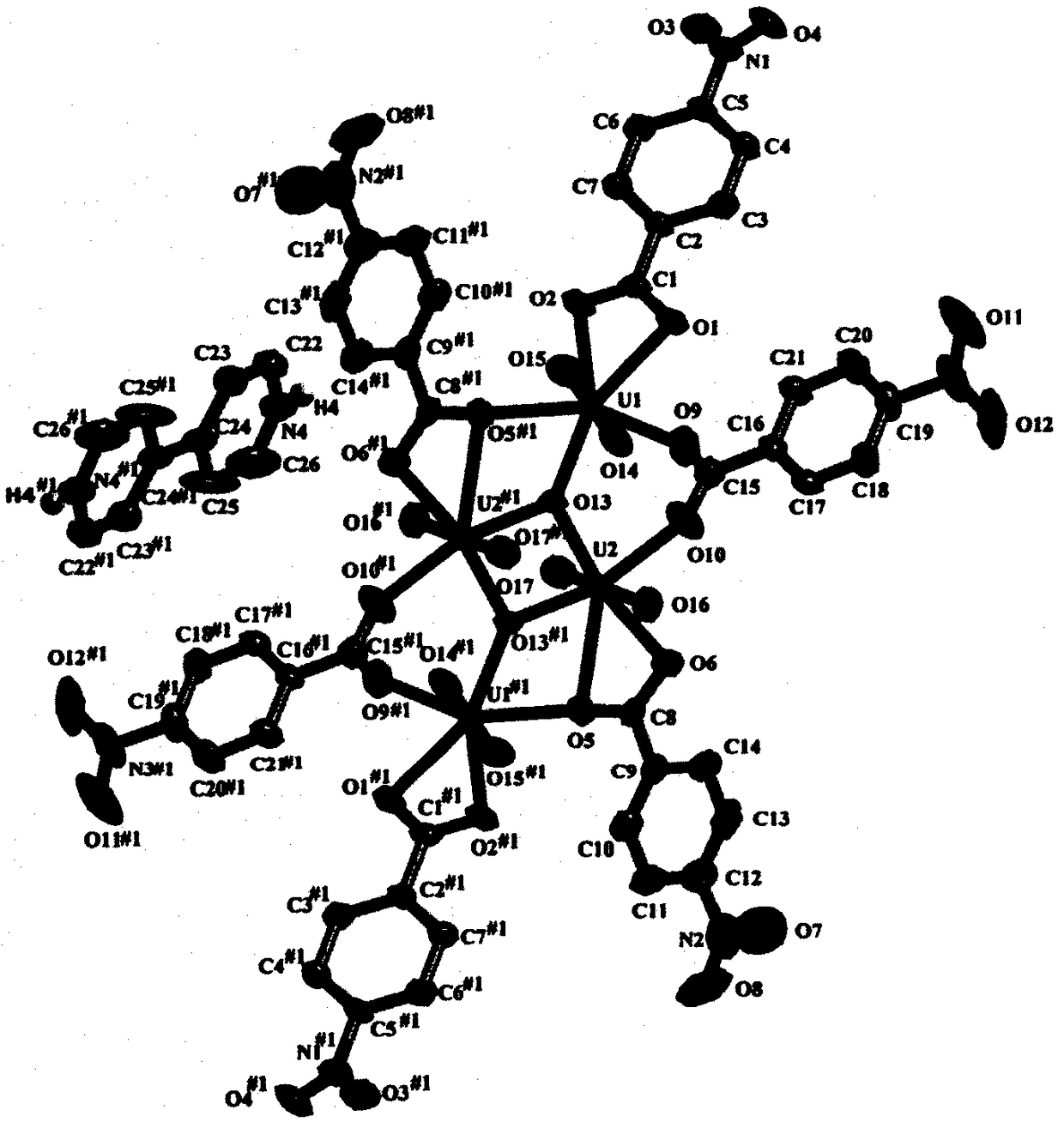
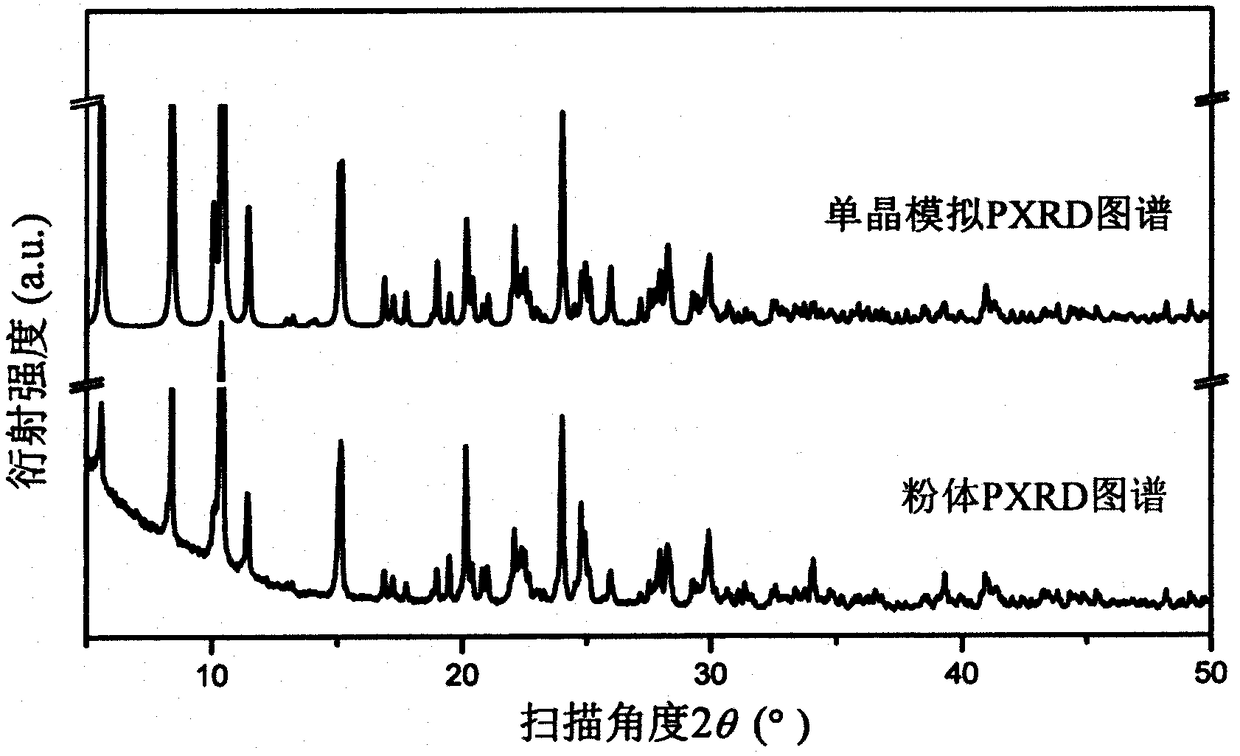
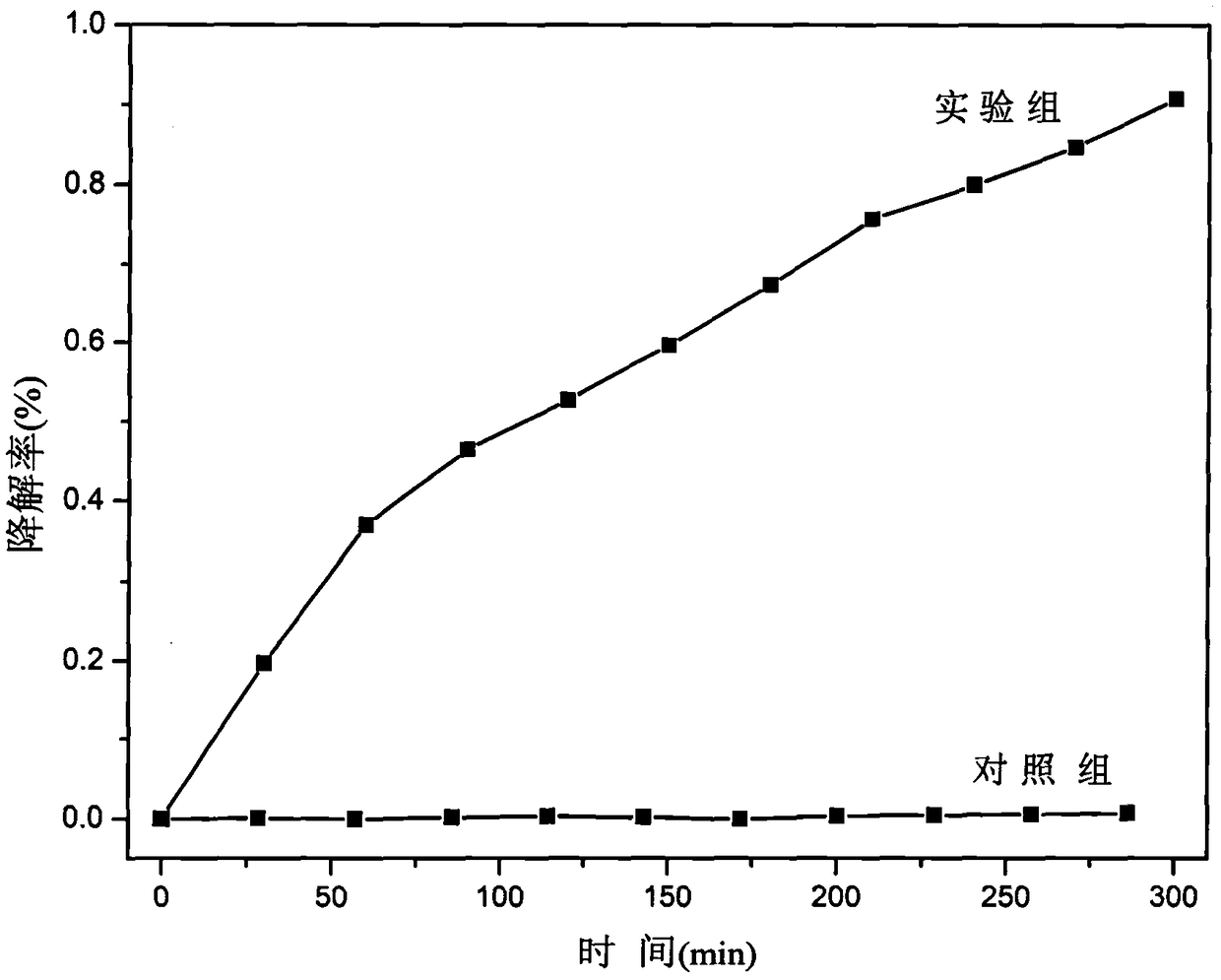
[0012] Weigh 0.25mmol uranyl acetate and dissolve in 5mL H 2 Dissolved in O to obtain a yellow solution, and weighed 0.5mmol of p-nitrobenzoic acid and 0.2mmol of 4,4'-bipyridyl into the above mixed solution and stirred, the solution became cloudy, and continued to stir for 30min, and the measured pH = 2.11, The mixed solution was transferred to a 23 mL polytetrafluoroethylene reactor liner, sealed, and kept at 140 °C for 72 h. Obtain yellow product after cooling, and this yellow product is molecular formula (H 2 -bipy)[(UO 2 ) 4 (μ 3 -O) 2 (Hp-nba) 6 ] A p-nitrobenzoic acid uranyl complex photocatalyst. Grind the yellow product into powder, weigh 48mg and place it in 60mL aqueous solution containing tetracycline hydrochloride with a concentration of 40mg / L, and place it in a photocatalyst reactor, turn on the stirrer, and irradiate it with a 300W xenon lamp under visible light. Xenon lamp, carry out photocatalytic degradation to it under visible light, take appropriate...
Embodiment 2
[0015] It is basically the same as Example 1, except that uranyl acetate is replaced by uranyl nitrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com