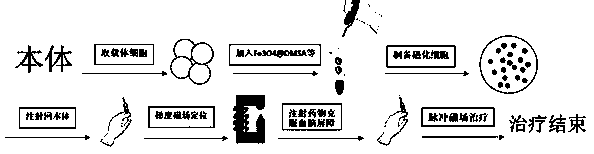
Method for controlling non-invasive brain entry of magnetic nano particles on basis of cell drug loading technology
A magnetic nanoparticle, technical control technology, applied in the field of combining nanotechnology and medicine, can solve problems such as high risk and high trauma, and achieve the effects of high biocompatibility, simple operation, and no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035]1) Preparation of magnetized cells (taking magnetized red blood cells as an example); blood collection and storage, the collected red blood cells are stored in blood preservation solution, and a large volume of ice saline is added, and centrifuged and washed according to individual differences and weeks of age Make appropriate adjustments to the rotation speed and time. Add appropriate concentration of hypotonic solution and MNPs solution into the mixture to make red blood cells in a hypotonic dilution environment; use appropriate concentration of resealing solution to restore the external environment of red blood cells to isotonic, so that red blood cells can be resealed; add appropriate concentration of PBS, disperse After red blood cells are centrifuged to remove free MNPs and exuded hemoglobin and other substances, magnetized red blood cells are obtained. The MNPs are Fe3O4@DMSA; the blood preservation solution is composed of sodium citrate, citric acid, glucose, sod...
Embodiment 1
[0040] Example 1: Preparation of Fe3O4 magnetized erythrocytes (taking mouse experiments as an example)
[0041] The preparation of Fe3O4 magnetized erythrocytes is carried out according to the existing mature method in our laboratory. The prepared magnetic erythrocytes have the characteristics of large loading capacity and high degree of magnetization.
[0042] The specific experimental process is as follows.
[0043] 1) Collect 0.1 mL of blood cells from the eye vein and store them in the pre-prepared blood preservation solution to prevent coagulation;
[0044] 2) Hypotonic dilution: After the hypotonic solution is added to the red blood cell solution, the volume of the red blood cells becomes larger due to swelling, and the appropriate concentration of the hypotonic solution ensures that the red blood cells will not rupture. The cell membrane is then accompanied by pores that allow the passage of macromolecules. Then 150 uL of MNPs solution was added to the mixture, and t...
Embodiment 2
[0046] Example 2: Aggregation of magnetized erythrocytes under the action of a magnetic field (taking mouse experiments as an example)
[0047] Tail vein injection of magnetized erythrocytes: first place the mouse in the holder, and make the tail of the mouse exposed from the small hole at the back of the holder. Then use the thumb and forefinger of the left hand to straighten the tail and keep it tight. Use a cotton ball dipped in alcohol to gently wipe the tail and moisten it so that the tail vein swells until it is clearly visible. Next, place the tail of the mouse over the index finger and secure it with the thumb and middle finger, and hold the 1 mL syringe in the right hand for needle insertion. When injecting, the left hand needs to hold the tail. The needle insertion position is generally selected to be 1 / 4 or 1 / 3 from the tip of the tail. The skin here is thinner, and the position of the blood vessels can be seen. Before injection, you can judge whether the needle ti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com