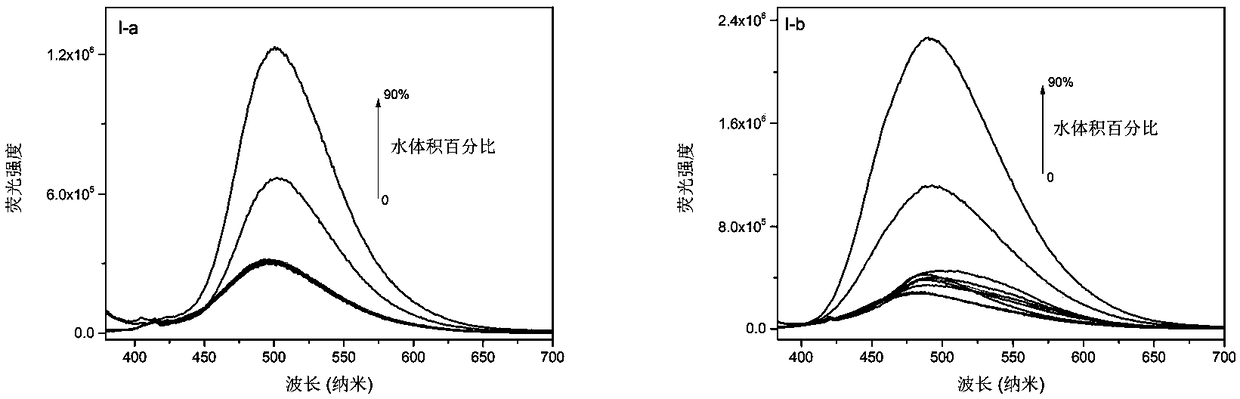
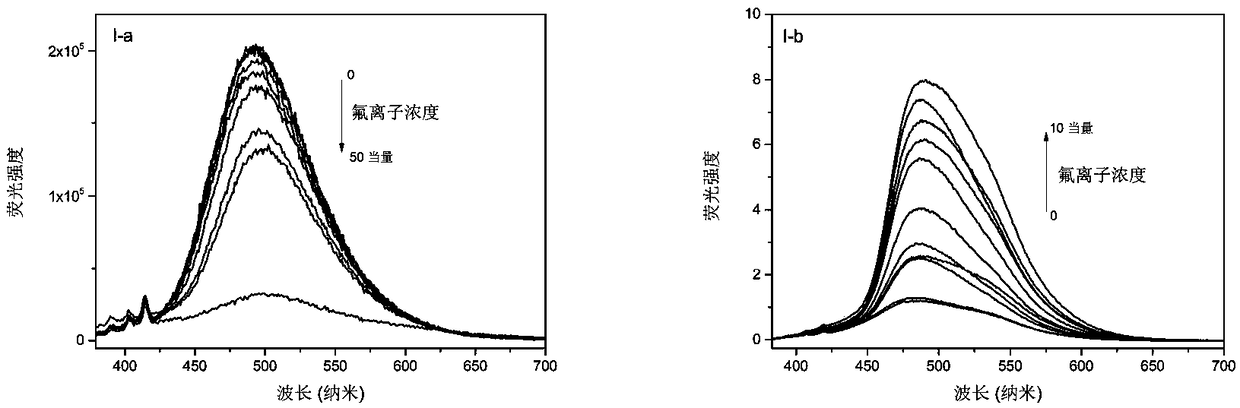
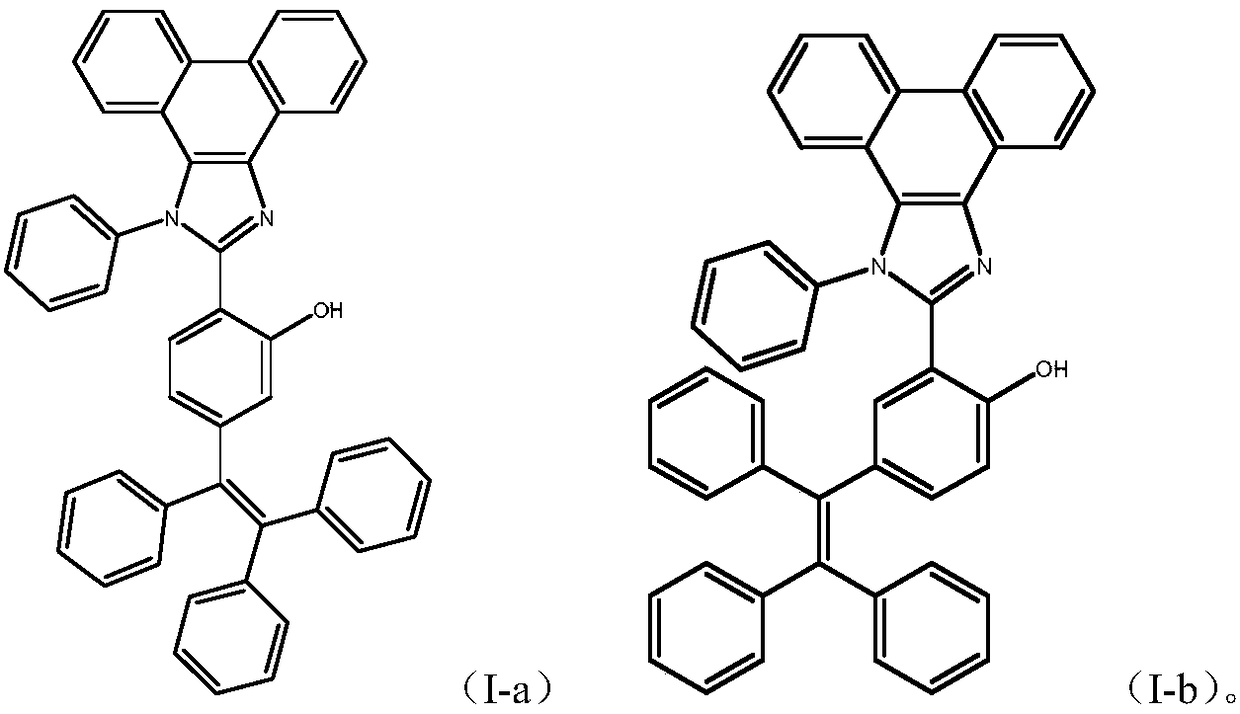
Phenanthroimidazole derivative with ESIPT (excited-state intramolecular proton transfer) and AIE (aggregation-induced emission) properties and preparation method and application thereof
A technology of phenanthroimidazole and derivatives, which is applied to phenanthroimidazole derivatives with ESIPT and AIE properties and their preparation and application fields, can solve problems such as low emission efficiency and concentration quenching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Synthesis of 4,4,5,5-tetramethyl-2-(1,2,2-triphenylethenyl)-1,3,2-dioxaborolane (VI)
[0037] Under nitrogen protection, 2-bromo-1,1,2-triphenylethylene (1.0 g, 3 mmol) represented by formula (IV) was dissolved in anhydrous tetrahydrofuran (20 mL) solvent, and 1.6M n-BuLi (3.0 mL, 7.5 mmol) was stirred for 1 h. Then isopropanol pinacol borate (2.15 mL, 10.7 mmol) represented by formula (V) was slowly added, and the system was heated to room temperature and reacted overnight. After the reaction was completed, the reaction was quenched with saturated aqueous ammonium chloride, extracted with deionized water and dichloromethane, and the obtained organic phase was added with anhydrous MgSO 4 After drying, concentrate under reduced pressure, and then separate and purify by column chromatography. The stationary phase is 300-400 mesh silica gel, and the mobile phase is a mixed solvent of petroleum ether / ethyl acetate (volume ratio 50:1). solution, the solvent was e...
Embodiment 2
[0039] Example 2 Synthesis of 5-bromo-2-(1-phenyl-1H-phenanthrene[9,10-d]imidazol-2-yl)phenol (VII-a)
[0040]Under nitrogen protection, 9,10-phenanthrenequinone (0.57g, 2.74mmol) represented by formula (II), 4-bromo-2-hydroxybenzaldehyde (0.5g, 2.5mmol) represented by formula (III-a), Ammonium acetate (0.96mg, 12.5mmol) was added to a 100mL two-necked flask, aniline (0.3mg, 3mmol) and 30mL of acetic acid were added, the temperature was raised to 120°C, and the reaction was carried out for 12h. After the reaction, cool the system to room temperature, add water and dichloromethane for extraction, combine the organic phases, dry overnight with anhydrous magnesium sulfate, remove the desiccant by suction filtration, evaporate the solvent under reduced pressure, add dichloromethane again to dissolve, Add crude silica gel to mix the sample, and pass through the column by chromatography. The eluent is a mixed solvent of petroleum ether and dichloromethane (volume ratio, 2:1), and fi...
Embodiment 3
[0042] Example 3 Synthesis of 4-bromo-2-(1-phenyl-1H-phenanthrene[9,10-d]imidazol-2-yl)phenol (VII-b)
[0043] The synthetic method is the same as Example 2, and the difference is that 4-bromo-2-hydroxybenzaldehyde shown in formula (III-a) is replaced by 5-bromosalicylaldehyde (201.02g / mol, 2.5mmol, 0.5g), the light yellow target product (VII-b) 1.02g was finally obtained, and the yield was 93%.
[0044] MS (ESI) characterization of 4-bromo-2-(1-phenyl-1H-phenanthrene[9,10-d]imidazol-2-yl)phenol found to be 466.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com