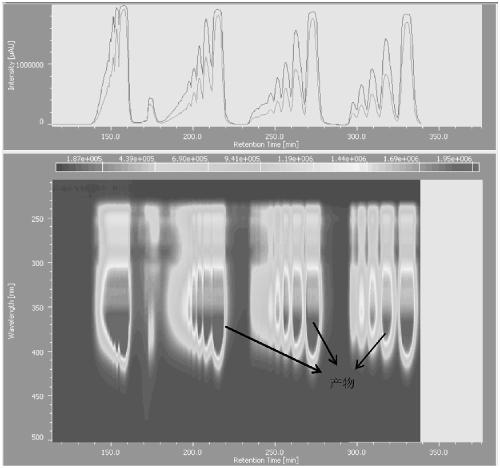
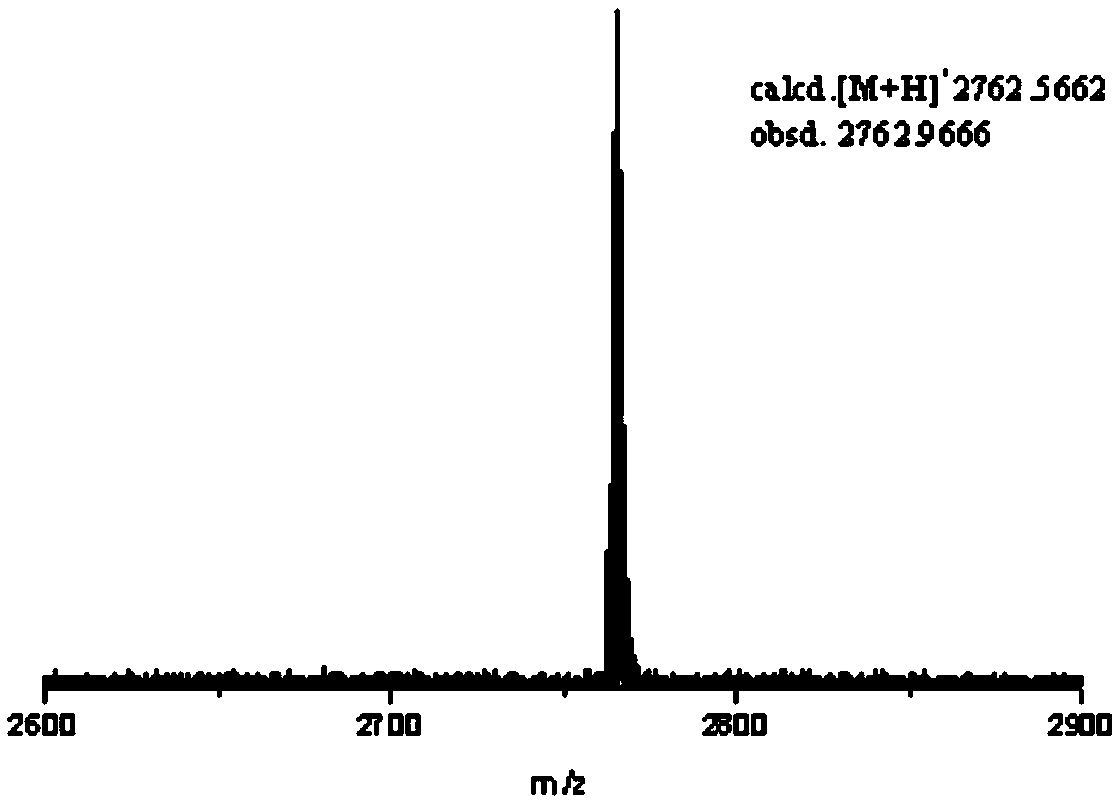
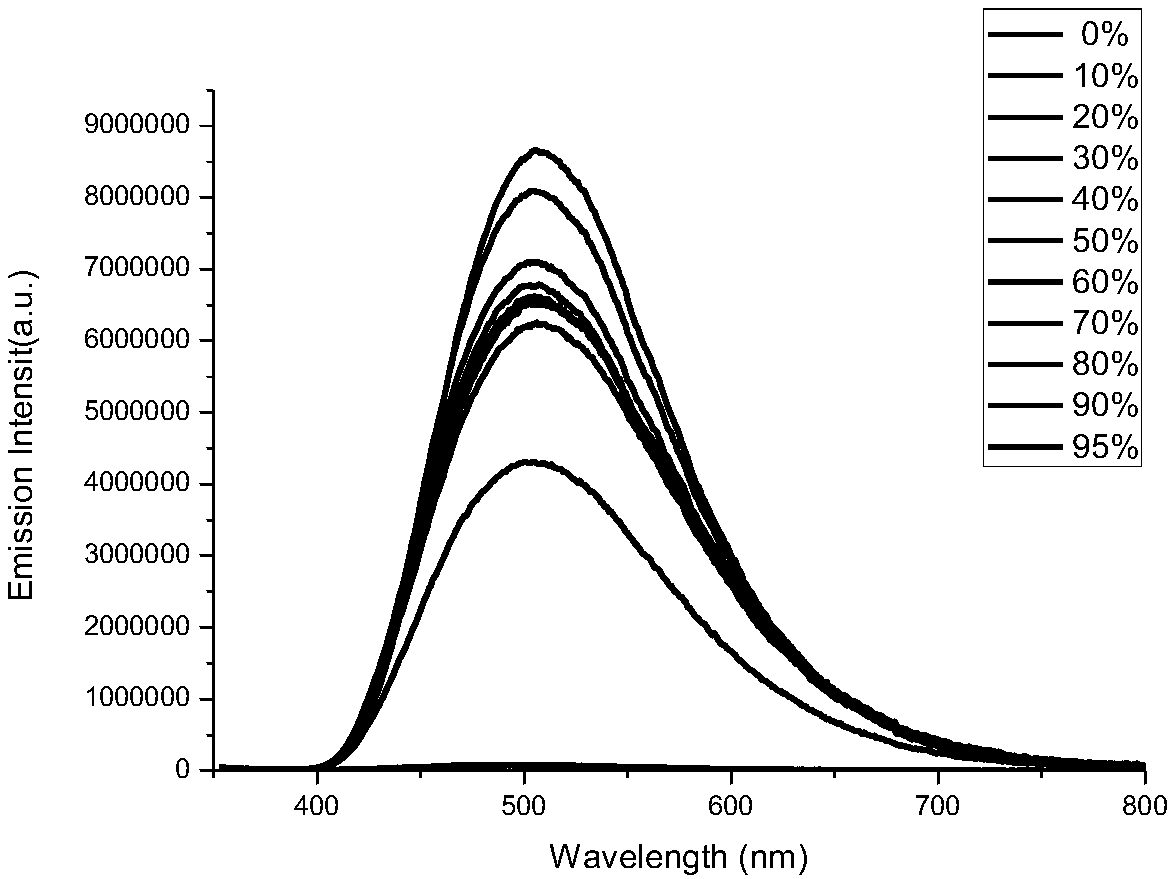
Rigid conjugate macrocyclic compound with AIE effect as well as preparation and application of rigid conjugate macrocyclic compound
A technology of conjugated macrocycles and compounds, which is applied in the field of rigid conjugated macrocycles and their preparation and application, and can solve the problems of decreased fluorescence emission efficiency, reduced degree of radiation decay, and restricted molecular movement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Synthesis of compound (4e):
[0074] NaH (8.4g, 0.352mol, 4.4eq.) was added to a 500mL oblique two-necked flask, and 250mL of freshly distilled anhydrous THF and 1-dodecanol (31g, 0.167mol, 2.1eq. ), stirred at room temperature, and then added 3-chloro-2-chloromethylpropene (10g, 0.08mol, 1eq.). Finally, the temperature was raised to 65°C and reacted overnight. After the reaction solution was cooled to room temperature, ethanol was slowly added to quench the reaction, THF was removed by rotary evaporation, and the reaction solution was washed with CH 2 Cl 2 Extracted, washed with water, dried over anhydrous magnesium sulfate, filtered, concentrated, and the crude product was purified by silica gel chromatography (eluent: PE / CH 2 Cl 2 =5 / 1), 25 g of colorless liquid was obtained, yield 75%. 1 HNMR (400MHz, CDCl 3 ):δ(ppm)5.14(s,2H),3.95(s,4H),3.39(t,J=6.6Hz,4H),1.62 –1.48(m,4H),1.25(s,36H),0.87( t,J=6.7Hz,6H).
Embodiment 2
[0076] Synthesis of compound (4d)
[0077] Add 4e (5.0g, 11.78mmol) into a 100mL oblique two-necked flask. After three times of argon replacement, add 80mL freshly distilled anhydrous THF under an argon atmosphere, and slowly add THF-dissolved BH at 0°C. 3 (1mol / L, 28mL), and after stirring rapidly for 3 hours, 18mL NaOH (3mol / L) solution was added. Stir vigorously for another 15 minutes, add 18 mL of H 2 o 2 (30%), after stirring overnight at room temperature, add K 2 CO 3 to saturation, the reaction solution was subjected to CH 2 Cl 2 Extracted, washed with water, dried over anhydrous magnesium sulfate, filtered, concentrated, and the crude product was purified by silica gel column chromatography to obtain 3.5 g of a colorless liquid with a yield of 79%. 1 H NMR (400MHz, CDCl 3 ): δ(ppm)3.78(d,J=5.1,2.4Hz,1H),δ3.76(d,J=5.0Hz,1H),δ3.68(d,J=6.1Hz,1H),3.62– 3.58(m,2H),3.52(m,J=9.4,6.0Hz,2H),3.47–3.25(m,4H),2.17(s,1H),1.55(d,J=10.2,3.9Hz,4H) ,1.46–1.03(m,36H),0.99–0.74(...
Embodiment 3
[0079] Synthesis of compound (4c)
[0080] Add 4d (2g, 4.5mmol, 1eq.) and TsCl (3.91g, 22.66mmol, 5eq.) into a 100mL oblique two-necked flask. After pumping argon three times, add 60mL of anhydrous CH under argon atmosphere. 2 Cl 2 , pyridine (5.3g, 67.5mmol, 15eq.), stirred rapidly at 25°C for 5 hours, and the reaction solution was passed through CH 2 Cl 2 Extraction, combined organic phase, the organic phase was washed with 1mol / L HCl first, then washed with distilled water, dried over anhydrous magnesium sulfate, filtered, concentrated, and the crude product was purified by silica gel chromatography (eluent: CH 2 Cl 2 ), 2.2 g of light yellow liquid was obtained, and the yield was 85%. 1 H NMR (400MHz, CDCl 3 ):δ(ppm)7.81(d,J=8.3Hz,2H), 7.36(d,J=8.0Hz,2H),4.12(d,J=5.6Hz,2H),3.46–3.23(m,8H) ,2.47(s,3H),2.20(q,J=11.5,5.8Hz,1H),1.54–1.40(m,4H),1.37–1.17(m,36H),0.90(t,J=6.8Hz,6H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com