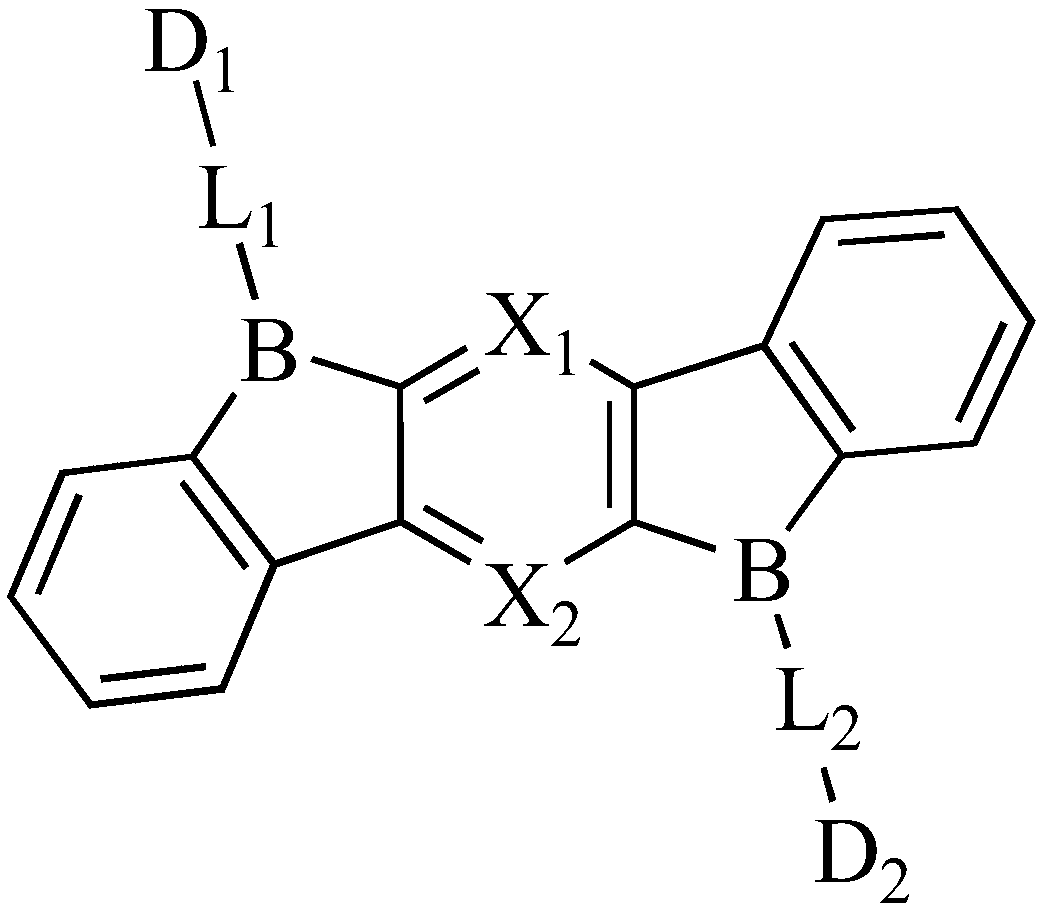
Boron heterocyclic compound and organic light emitting display device
A light-emitting display and compound technology, applied in the field of organic electroluminescent materials, can solve the problems of short life, high cost of phosphorescent devices, poor stability, etc., and achieve the effects of increasing energy levels, weakening charge transfer, and shortening the conjugate length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088]
[0089] Add 10g (20.50mmol) of the compound 1,4-dibromo-2,5-diiodopyridine, 9.06g (45.11mmol) of dibromophenylboronic acid, and 5.67g (41mmol) of potassium carbonate into a three-necked flask in turn, and add 100mL of toluene solution, stir well. Nitrogen was replaced three times, and 0.1 g of palladium acetate was added under nitrogen atmosphere, and nitrogen was replaced three times after the addition. Afterwards, it was stirred for 12 h under nitrogen atmosphere. Cool to room temperature at the end of the reaction, and use 200mL saturated NaHSO 3 The reaction was quenched, and the organic phase was extracted with dichloromethane (150 mL), and the organic phase was then washed with saturated NaHSO 3 Extract twice, and finally extract once with saturated saline. The collected organic phase was added with anhydrous magnesium sulfate, stirred, filtered, and the collected filtrate was rotary evaporated to remove the solvent. The product was purified by column chro...
Embodiment 2
[0105]
[0106] Add 3.1 g (9.57 mmol) of 4-bromophenyldiphenylamine into the reaction flask, add diethyl ether (50 mL) to dissolve, and replace with nitrogen three times. Cool down to -78°C, and when the temperature reaches, control the temperature below -65°C and slowly add n-BuLi 4.21mL (2.5M, 10.52mmol) dropwise, and stir for 30min after the dropwise addition is complete. Then 3.9 g (9.57 mmol) of monomer 103 was dissolved in 60 mL of toluene, and then slowly added dropwise to the reaction liquid, and after the completion of the dropwise addition, it was naturally raised to room temperature for 6 h. After the reaction was completed, ice water (100 mL) was added to quench the reaction. Then add DCM (80mL×2) for extraction, and finally extract once with saturated brine. The collected organic phase was rotary evaporated to obtain a yellow oil. The product was purified by column chromatography (mobile phase n-hexane:dichloromethane=10:1) to obtain 2.46 g (4.30 mmol) of lig...
Embodiment 3
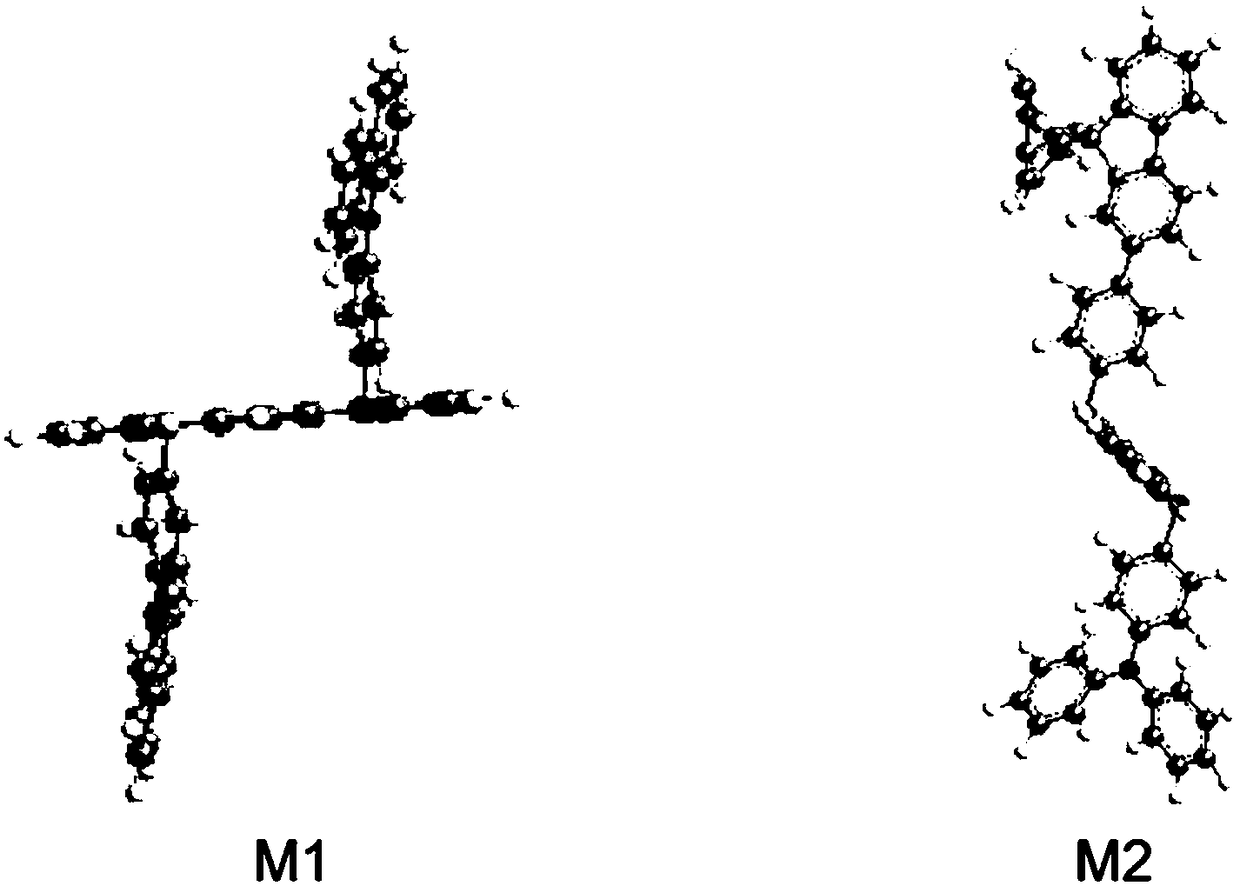
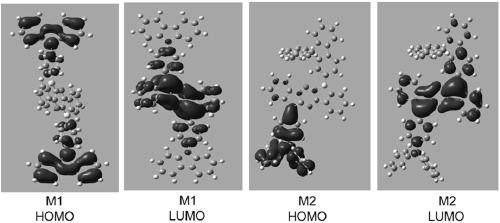
[0114] The chemical structure of the boron heterocyclic compound prepared in embodiment 1 and embodiment 2 is simulated by Gaussian software, and the 3D solid model obtained by simulation is shown in figure 1 . Depend on figure 1 It can be seen that both the donor and acceptor groups are almost perpendicular to the boracarbazole, and the dihedral angles are 88.15 and 62.17, respectively. The highly twisted structure is beneficial to reduce the △E ST , improve the ability to cross the reverse gap.
[0115] Similarly, the boron heterocyclic compounds M1 to M4, M1', M2' were simulated using Gaussian software, where M1' and M2' are compounds obtained by replacing the pyrazine rings in M1 and M2 molecules with benzene rings. The simulation results are shown in Table 1.
[0116] Table 1
[0117]
[0118] It can be seen from Table 1 that the HOMO of the molecules of compounds M1-M4 is about 5.1ev-5.5ev, the energy range between the singlet state and the triplet state is about ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com