Phenylglycyl amino acid benzyl ester modified curcumin, synthesis, activity and application thereof
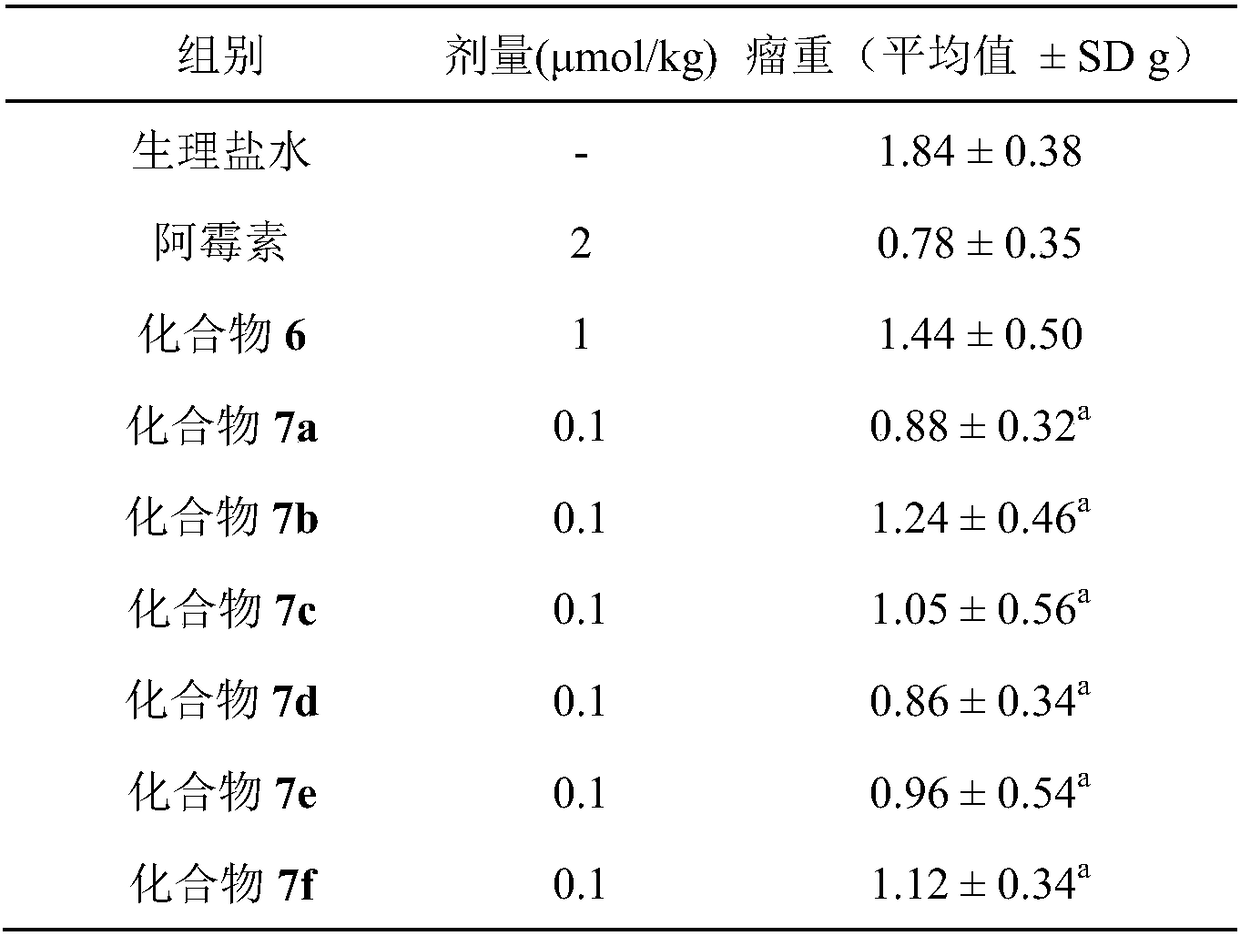
A technology of oxyacetyltheanyl and oxyacetylbenzyl ester, applied to 1--7--1,6-heptadiene-3,5-dione, and its application in the preparation of anti-tumor drugs and anti-inflammatory drugs It can solve the problems of affecting the effect of chemotherapy, unsatisfactory clinical efficacy of tumor chemotherapy, and no anti-inflammatory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
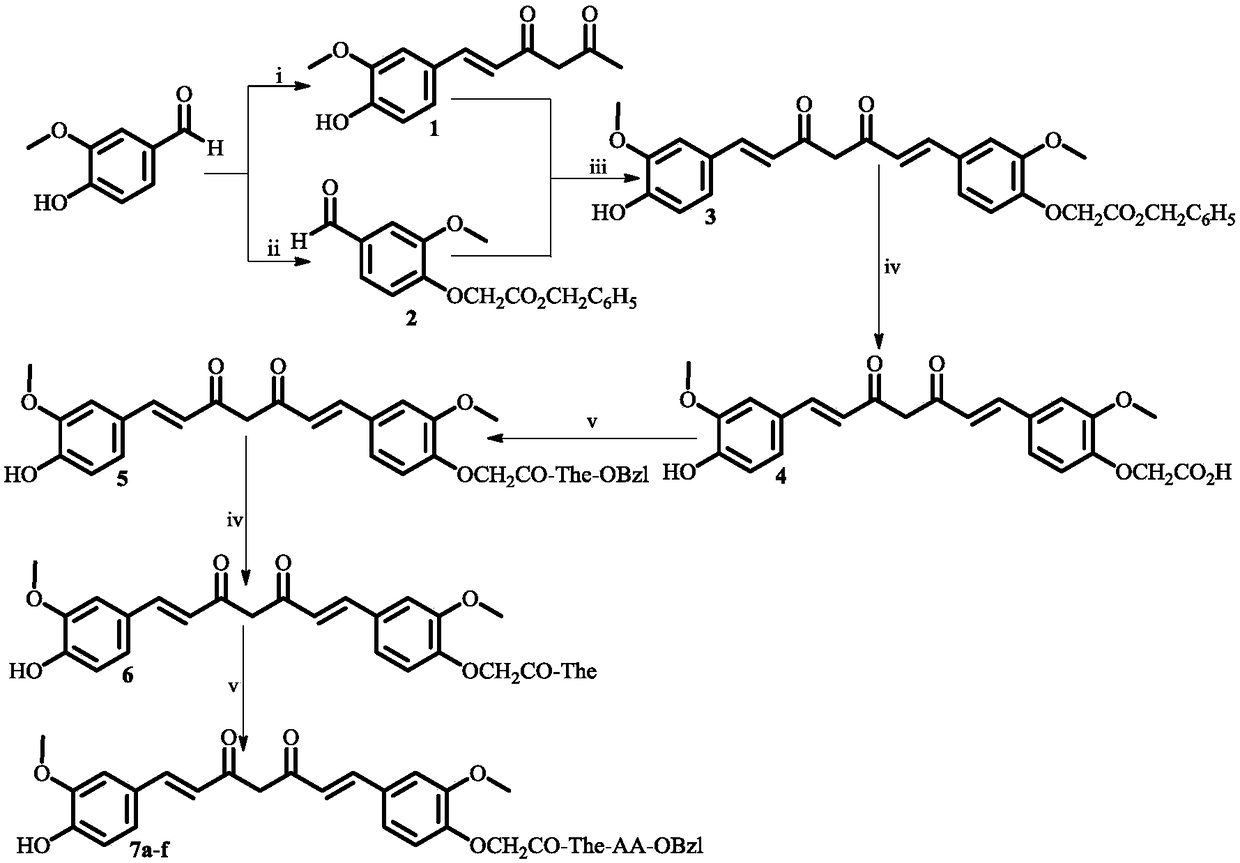
[0017] Example 1 Preparation of 6-(4-hydroxyl-3-methoxyphenyl)-5,6-hexene-2,4-dione (1)
[0018] Reflux 45.0 mL (437.7 mmol) of acetylacetone, 21.0 g (301.6 mmol) of boron oxide and 150.0 mL of anhydrous ethyl acetate at 60° C. for 1 h. Then, 22.5 g (148.0 mmol) of vanillin and 41 mL (293.0 mmol) of tributyl borate were added thereto. The reaction mixture was stirred at 70 °C for 30 min. Continue to add a solution of 15 mL (205.1 mmol) of n-butylamine and 135 mL of ethyl acetate within 30 min. The mixture was stirred at 100°C for 3 h, then cooled to room temperature, and 150 mL of hydrochloric acid (1M) was added dropwise thereto. The mixture was stirred at 50°C for 30 min, allowed to stand, and the layers were fully separated, and the aqueous layer was extracted three times with ethyl acetate. The combined ethyl acetate layers were washed with saturated NaCl solution until neutral, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated to dryness unde...
Embodiment 2
[0019] Example 2 Preparation of 3-methoxy-4-(oxygen-2-acetylbenzyl)benzaldehyde (2)
[0020] Dissolve 10 g (65.8 mmol) of vanillin in 100 mL of anhydrous tetrahydrofuran. To this solution was added portionwise 10.9 g (79.0 mmol) potassium carbonate and stirred for 3 h. Then, 9.3 mL benzyl bromoacetate was added dropwise to the solution, stirred at room temperature for 48 h, and TLC monitoring (petroleum ether / ethyl acetate=3 / 1) showed that the reaction was complete. The reaction mixture was filtered, and the filtrate was concentrated under reduced pressure. The residue was washed with 100 mL of ether and allowed to stand for 12 h, then poured off the ether and washed with 10 mL of ether three times to obtain 15.4 g (78%) of the title compound as a colorless solid. ESI-MS(m / e): 301[M+H] + ; 1 H NMR (300MHz, DMSO-d 6 ):δ / ppm=9.86(s,1H),7.50(dd,J 1 =8.4Hz,J 2 =1.8Hz,1H),7.44(d,J=1.8Hz,1H),7.39(s,5H),7.11(d,J=8.4Hz,1H),5.21(s,2H),5.03(s,2H ), 3.84(s,3H).
Embodiment 3
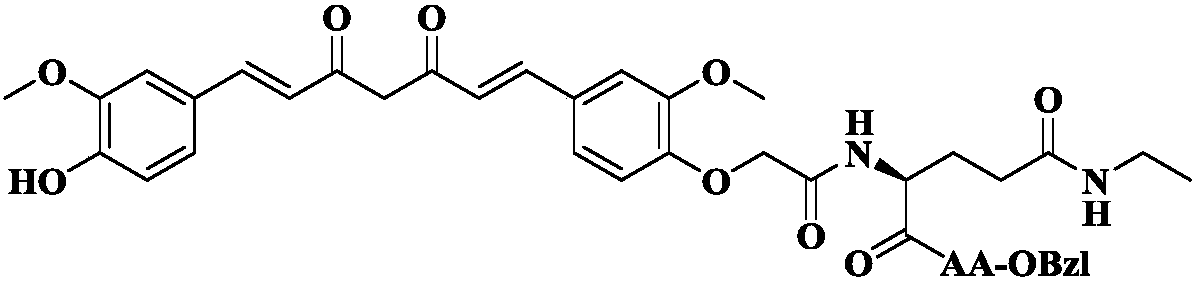
[0021] Example 3 Preparation of 1-(4-hydroxyl-3-methoxyphenyl)-7-(4-oxoacetylbenzyl-3-methoxyphenyl)-1,6-heptadiene-3, 5-diketone(3)
[0022] 5.55 g (23.7 mmol) of 6-(4-hydroxy-3-methoxyphenyl)-5,6-hexene-2,4-dione (1), 0.83 g (11.9 mmol) of boron oxide and 100 mL of acetic acid The ethyl ester suspension was refluxed at 70°C for 1h. After that, it was concentrated under reduced pressure. The residue was dissolved with 100 mL of anhydrous DMF. To the resulting solution were added 10.67 g (35.6 mmol) of 3-methoxy-4-(oxy-2-acetylbenzyl)benzaldehyde (2) and 11.15 mL (41.0 mmol) of tributyl borate. The resulting solution was stirred at 80 °C for 30 min. Afterwards, 0.98 mL (6.4 mmol) of n-butylamine was added dropwise in 4 times for 1 h, and the resulting solution was stirred at 80° C. for 3 h. After that, 200 mL of 10% aqueous acetic acid solution preheated to 60° C. was added thereto. The resulting solution was stirred for an additional 1 h at 80 °C. The reaction mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com