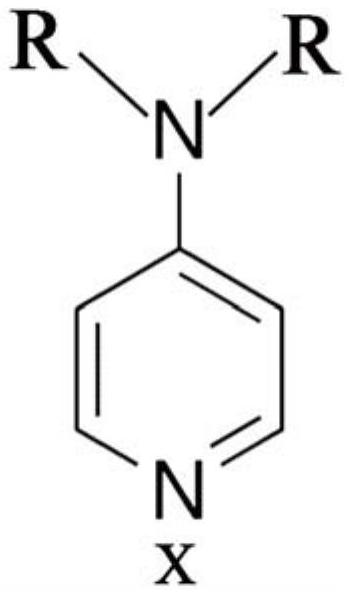
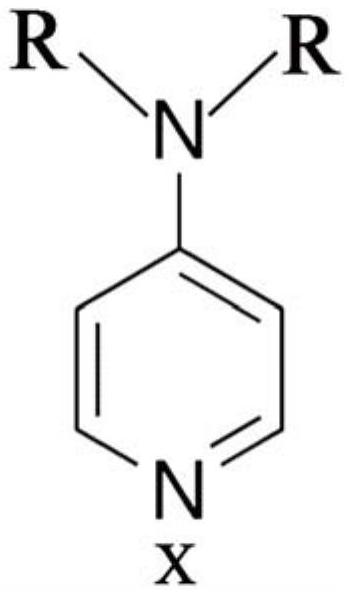
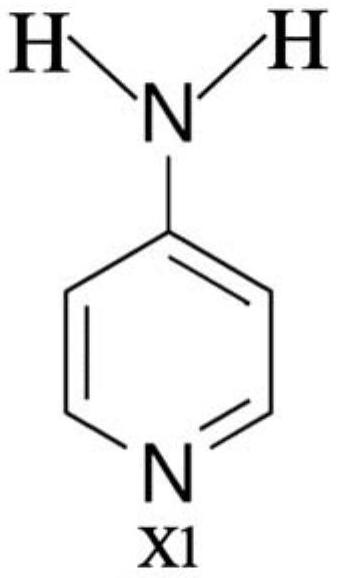
A kind of oral antithrombotic drug and its application
A technology for drugs and pharmaceutical excipients, which can be used in drug combinations, pharmaceutical formulations, blood diseases, etc., and can solve problems such as unclear research and complex thrombosis mechanisms.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 antithrombotic oral tablet
[0039] Prescription: 120 mg of captopril, 60 mg of compound (X1), 1200 mg of microcrystalline cellulose, 1200 mg of lactose, 120 mg of low-substituted hydroxypropyl cellulose, 24 mg of corn starch, 24 mg of magnesium stearate and water.
[0040]preparation:
[0041] (1) Take the prescribed amount of captopril, compound (X1), microcrystalline cellulose, lactose, low-substituted hydroxypropyl cellulose, cornstarch and magnesium stearate and pulverize them through an 80-mesh sieve;
[0042] (2) Captopril, compound (X1), microcrystalline cellulose, lactose, and low-substituted hypromellose powder are mixed;
[0043] (3) Prepare corn starch powder into 10% starch slurry, mix it with the powder mixture obtained in step (2) to prepare soft material, pass through a 20-mesh sieve for granulation, dry at 60°C, pass through a 18-mesh sieve for granulation, and harden Magnesium fatty acid, mixed evenly, compressed into tablets, each table...
Embodiment 2
[0044] Embodiment 2 antithrombotic oral tablet
[0045] Prescription: 120 mg of captopril, 40 mg of compound (X1), 1200 mg of microcrystalline cellulose, 1200 mg of lactose, 120 mg of low-substituted hydroxypropyl cellulose, 24 mg of corn starch, 24 mg of magnesium stearate and water.
[0046] Preparation: (1) Take the prescribed amount of captopril, compound (X1), microcrystalline cellulose, lactose, low-substituted hydroxypropyl cellulose, corn starch and magnesium stearate, and grind them through an 80-mesh sieve;
[0047] (2) Captopril, compound (X1), microcrystalline cellulose, lactose, and low-substituted hypromellose powder are mixed;
[0048] (3) Prepare corn starch powder into 10% starch slurry, mix it with the powder mixture obtained in step (2) to prepare soft materials, pass through a 20-mesh sieve for granulation, dry at 50°C, pass through a 18-mesh sieve for granulation, and harden Magnesium fatty acid, mixed evenly, compressed into tablets, each tablet contains...
Embodiment 3
[0049] Embodiment 3 antithrombotic oral tablet
[0050] Prescription: 120 mg of captopril, 60 mg of compound (X2), 1200 mg of microcrystalline cellulose, 1200 mg of lactose, 120 mg of low-substituted hydroxypropyl cellulose, 24 mg of corn starch, 24 mg of magnesium stearate and water.
[0051] preparation:
[0052] (1) Take the prescribed amount of captopril, compound (X2), microcrystalline cellulose, lactose, low-substituted hydroxypropyl cellulose, cornstarch and magnesium stearate and pulverize them through an 80-mesh sieve;
[0053] (2) Captopril, compound (X2), microcrystalline cellulose, lactose, and low-substituted hypromellose powder are mixed;
[0054] (3) Prepare corn starch powder into 10% starch slurry, mix it with the powder mixture obtained in step (2) to prepare soft material, pass through a 20-mesh sieve for granulation, dry at 60°C, pass through a 18-mesh sieve for granulation, and harden Magnesium fatty acid, mixed evenly, compressed into tablets, each tabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com