Method for extracting lithium and iron phosphate from lithium iron phosphate waste
A technology of lithium iron phosphate and iron phosphate, which is applied in the field of waste recycling, can solve the problems of general iron phosphate product indicators, many process flows, and poor removal effect of positive electrode aluminum, and achieve low cost, short process flow, and good The effect of economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
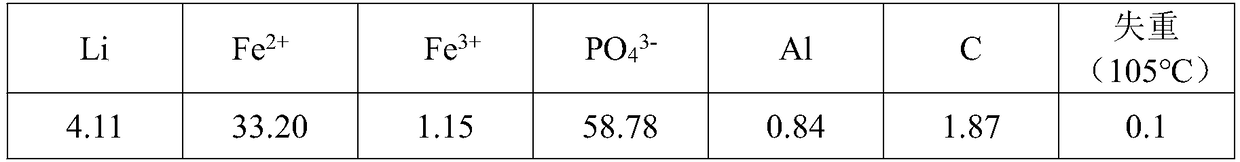
[0053] (1) Weigh 200g of lithium iron phosphate powder. The data is as follows:
[0054] Table 1 Lithium iron phosphate powder data, %
[0055]
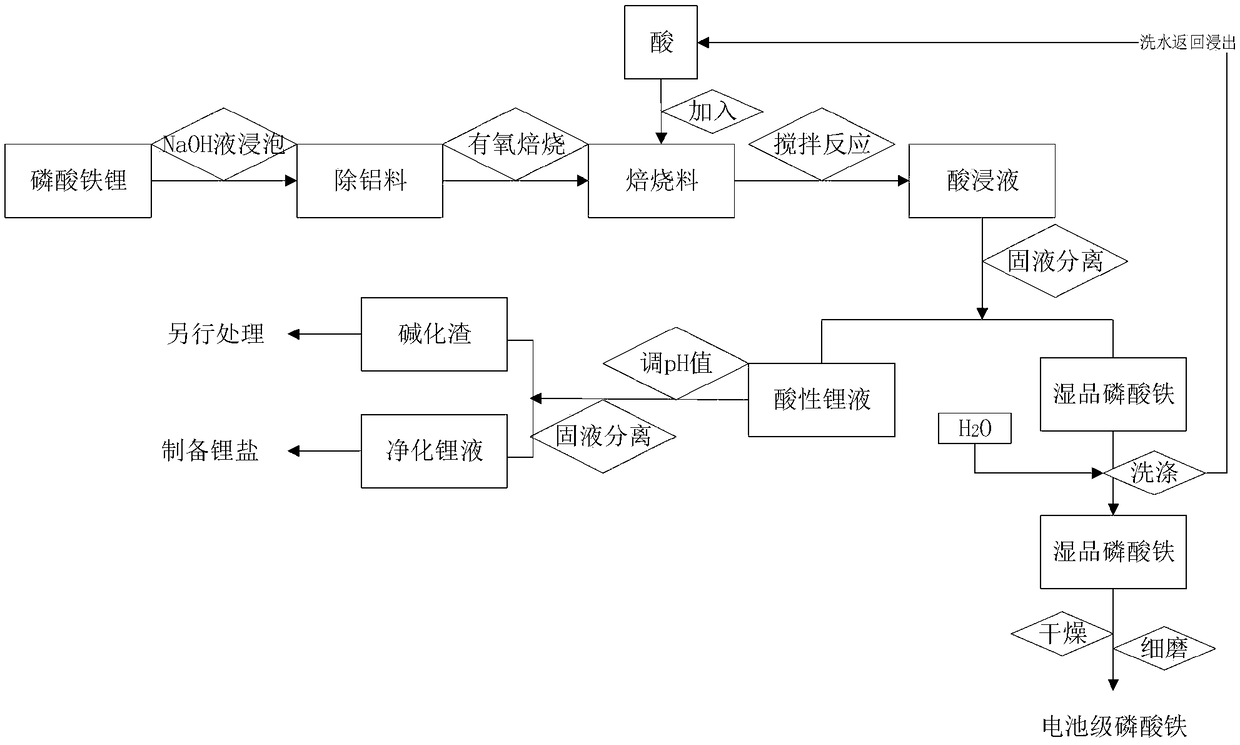
[0056] Add 500mL of 1moL / L NaOH solution and soak for 30min. After the violent reaction is over, separate the solid and liquid and wash. The obtained alkaline aluminum liquid is treated separately, and the obtained solid is aluminum-removing material.
[0057] (2) Carry out the aerobic roasting reaction at 480° C. for the aluminum removal material obtained in step (1). The time for the aerobic roasting reaction is 0.5 h, and the oxygen volume fraction is controlled to 20%. After the reaction is completed, the data of the roasted material obtained are as follows:
[0058] Table 2 roasted material data, %
[0059] Li
[0060] *Note: ND means not detected, the same below.
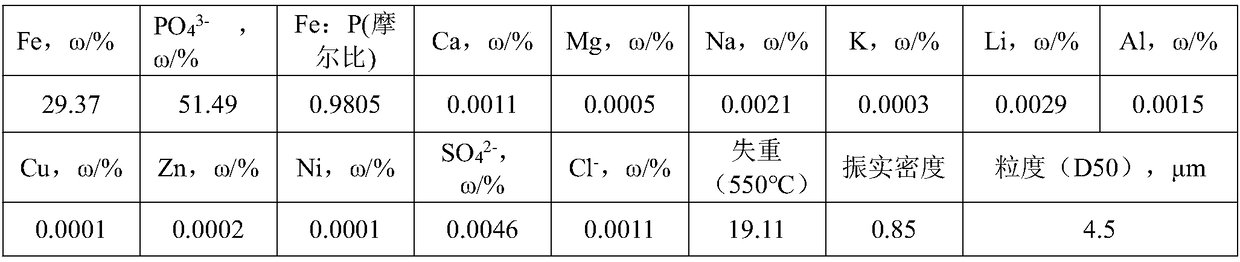
[0061] (3) Cool the roasted material obtained in step (2) to below 100°C, add 200mL of water, slowly add 36mL of concentrated sulfuric acid, stir fo...
Embodiment 2
[0074] (1) Weigh 500g of lithium iron phosphate powder. Data are as follows:
[0075] Table 6 Lithium iron phosphate powder data, %
[0076]
[0077] Add 1000mL of 1.5moL / L NaOH solution, soak for 120min, and after the violent reaction is over, separate the solid and liquid and wash. The obtained alkaline aluminum liquid is treated separately, and the obtained solid is aluminum-removing material.
[0078] (2) Carry out aerobic roasting reaction at 800° C. for the aluminum-removing material obtained in step (1). The time for the aerobic roasting reaction is (1 h), and the oxygen volume fraction is controlled to 50%. After the reaction is completed, the roasted material data obtained are as follows:
[0079] Table 7 roasted material data, %
[0080] Li
[0081] (3) Cool the calcined material obtained in step (2) to below 100°C, add 650mL of water, add 350mL of hydrochloric acid (30%), stir and react for 60min, control the pH end point to 1.8, and obtain the pickl...
Embodiment 3
[0094] (1) Weigh 1000g of lithium iron phosphate powder. The data is as follows:
[0095] Table 11 Lithium iron phosphate powder data, %
[0096]
[0097] Add 2000mL of 2moL / L NaOH solution and soak for 180min. After the violent reaction is over, separate the solid and liquid and wash. The obtained alkaline aluminum liquid is treated separately, and the obtained solid is aluminum-removing material.
[0098] (2) The aluminum-removing material obtained in step (1) is subjected to an aerobic roasting reaction at 1050°C. The time for the aerobic roasting reaction is 90 minutes, and the oxygen volume fraction is controlled to 60%. After the reaction is completed, the data of the roasted material obtained are as follows:
[0099] Table 12 roasted material data, %
[0100] Li
[0101] (3) Cool the calcined material obtained in step (2) to below 100°C, add 2100mL of nitric acid (3mol / L), stir and react for 120min, and control the pH end point to 3.0 to obtain an acid d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com