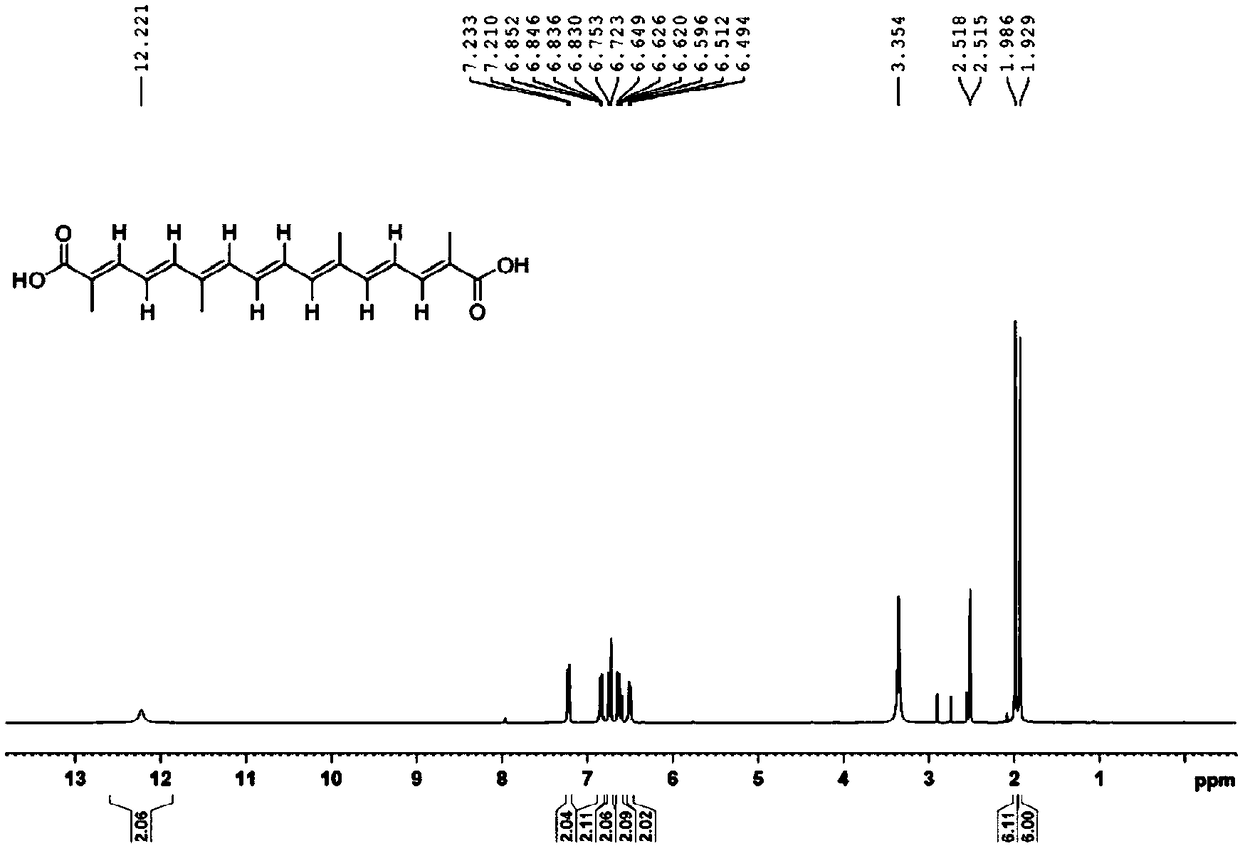
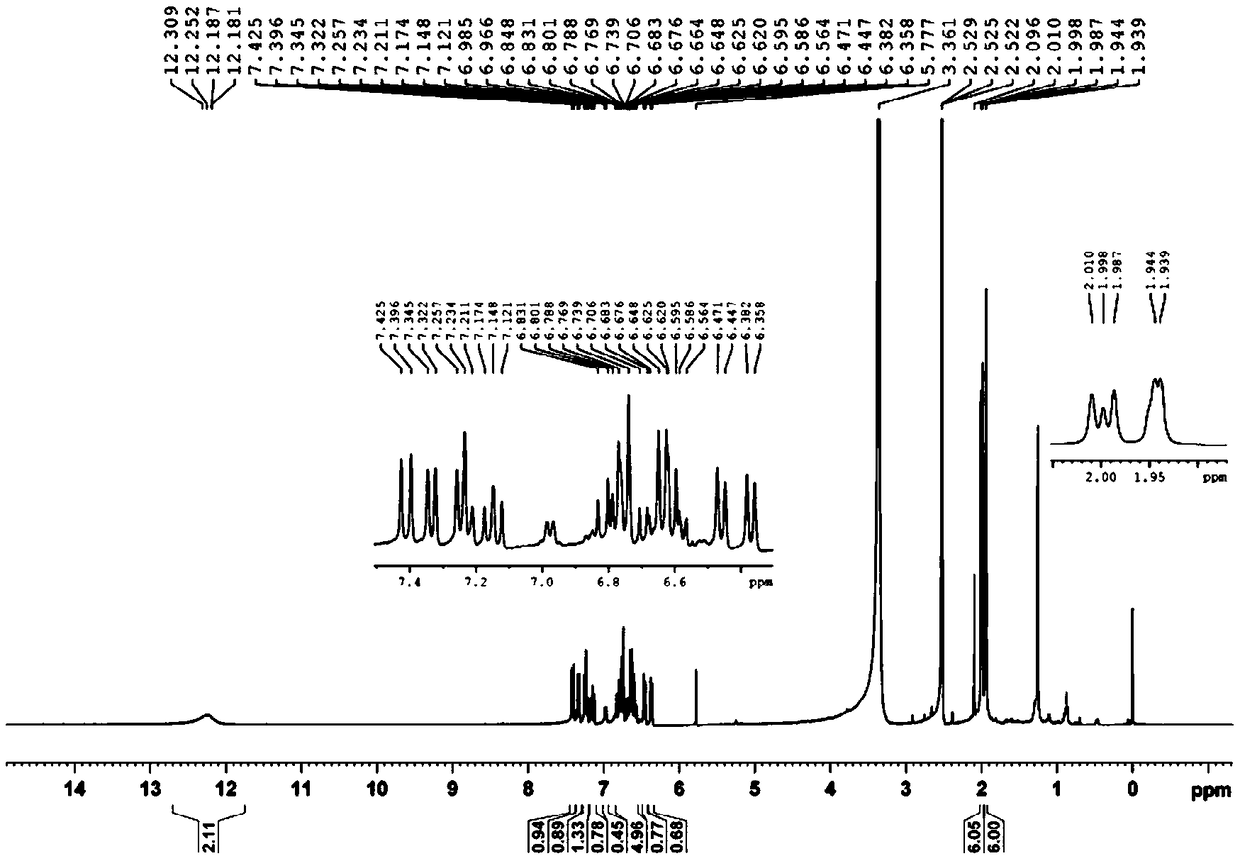
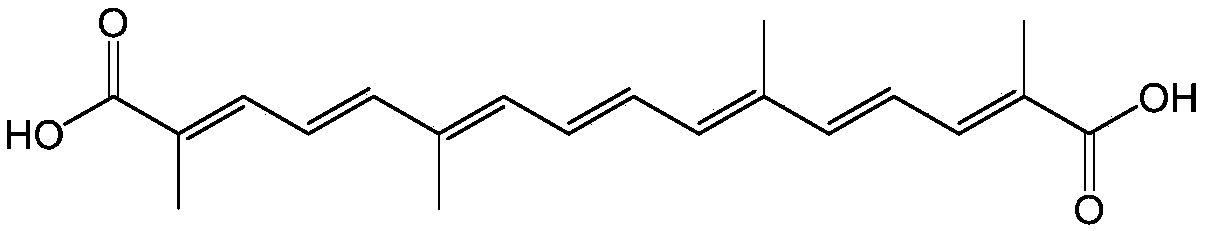
Separation method of cis-trans isomerism crocetin
A technology of crocetin and cis-trans isomerization, applied in the field of natural medicinal chemistry, can solve the problems of large ethanol consumption, insoluble crocetin in ethanol, and large alkali consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (1) Get 20g of crocetin crude product (the E-crocetin content is 62% after detection, Z-crocetin content is 8.3%) and 1500mL pure water are poured into beaker, regulate pH with sodium hydroxide alkali and be 11, heating promotes crocetin Dissolve the crude safflower acid, filter and discard the contents while it is hot;
[0072] (2) Slowly drop 10 mL of 5% calcium chloride into the filtrate, heat and crystallize at 60° C. for 4 h;
[0073] (3) filter;
[0074] (4) The filter residue is rinsed twice with 75% ethanol, then acidified with 200mL of 1% citric acid, kept at 60°C for 2h, filtered, washed with pure water, and the filter residue is vacuum-dried to obtain all-trans crocetin 12.1g (through HPLC detection, the purity is 97%);
[0075] (5) Adding 5% citric acid to the filtrate of (3) until the pH is 4, insulated for 2h, filtered, rinsed with pure water, and the filter residue was vacuum-dried to obtain cis-crocetin 1.62g (detected by HPLC, the purity was 94%) ). ...
Embodiment 2
[0077] (1) Take 100g of gardenia yellow (color value is 405) and 800mL of pure water in a beaker, heat to 70°C to dissolve completely; slowly add 50mL of 20% sodium hydroxide into the gardenia yellow solution, keep stirring, 60 Insulate at ℃ for 30 minutes; add 10% hydrochloric acid dropwise to the reaction solution until the pH is 4; insulate for 1 hour to age the precipitate; filter with suction, wash the filter residue twice with pure water, and the dark red solid (after drying, the crude crocetin acid ).
[0078] (2) Transfer the dark red solid in (1) and 1000 mL of pure water into a beaker, adjust the pH to 10-11 with 10% sodium hydroxide, heat to promote dissolution, and filter to remove insoluble matter;
[0079] (3) Slowly add 4mL 5% CaCl 2 Solution, keep warm at 60°C for 4h;
[0080] (4) filter;
[0081] (5) The filter residue is rinsed twice with 80% ethanol, then acidified with 150mL of 1% citric acid, kept at 60°C for 2h, filtered, rinsed with pure water, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com