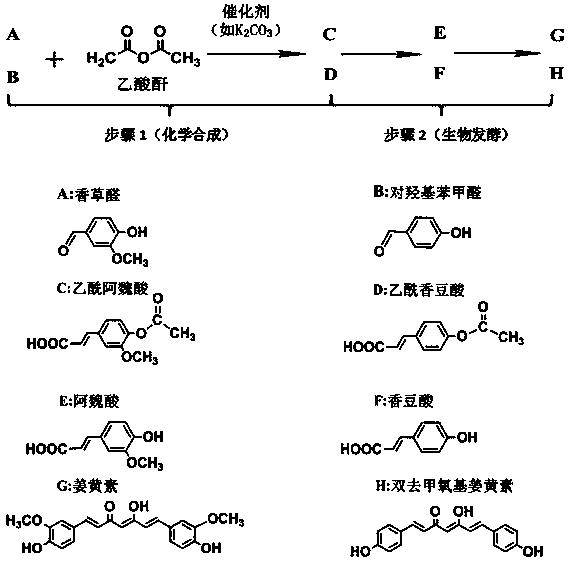
Method for preparing curcumin from vanillin and related analogues
A technology of analogs and vanillin, which is applied in the direction of microorganism-based methods, biochemical equipment and methods, and the preparation of organic compounds, can solve the problems of high energy consumption, single substrate price, and high pollution in chemical synthesis, and achieve improved The effect of product utilization efficiency, saving reaction time, and simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] 1. Chemical reverse synthesis of acetylferulic acid from vanillin as a substrate for biological fermentation:
[0051] (1) Combine vanillin and K 2 CO 3 Dry at about 50°C to constant weight.
[0052] (2) Add 1g of dry vanillin and K 2 CO 3 1.27g was stirred and mixed evenly, after stirring evenly, 6.7 mL of acetic anhydride was added into the flask, and the oil bath was 160°C, and reacted for 8 hours.
[0053] (3) After the reaction, pour the brown-yellow solution produced in step (2) into a beaker filled with about 30 g of crushed ice while it is still hot, to terminate the reaction, and then separate into an oil layer and a water layer.
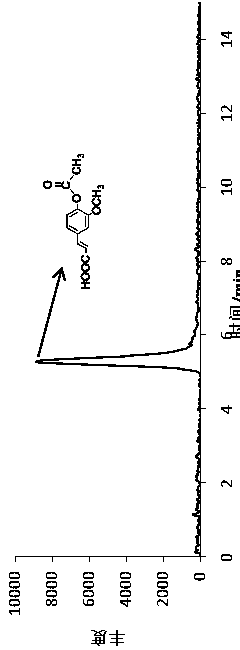
[0054] (4) Freeze the beaker overnight to separate the light yellow powdery substance, filter the product with suction, wash the filter cake until the washing liquid has no sour taste; dry the washed product at 50°C to obtain 1.35 g of the dried product, the product is mainly The ingredient is acetyl ferulic acid.
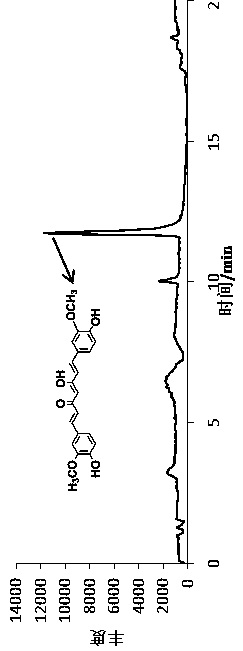
[0055] 2. P...
Embodiment 2
[0064] Other steps are with embodiment 1, but K in step 1 2 CO 3 Change to Na 2 CO 3 , the added amount is 0.98g.
Embodiment 3
[0066] Other steps are with embodiment 1, but K in step 1 2 CO 3 Change to CH 3 COONa, the added amount is 0.76g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com