A kind of tetraphenylene isomer with aggregation-induced luminescence and supramolecular polymerization properties, preparation method and application thereof
A technology of aggregation-induced luminescence and tetraphenylethylene, which is used in organic chemistry methods, luminescent materials, material excitation analysis, etc., to achieve excellent supramolecular polymerization ability, ease application difficulty, and easy synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
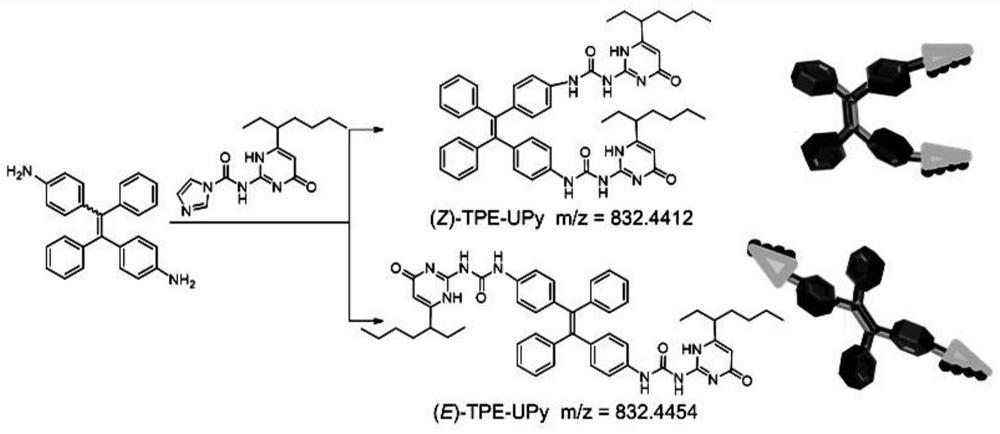
[0043] The present invention also provides a method for preparing tetraphenylethylene isomers with aggregation-induced luminescence and supramolecular polymerization properties, characterized in that: the preparation method includes the following chemical reactions:
[0044]
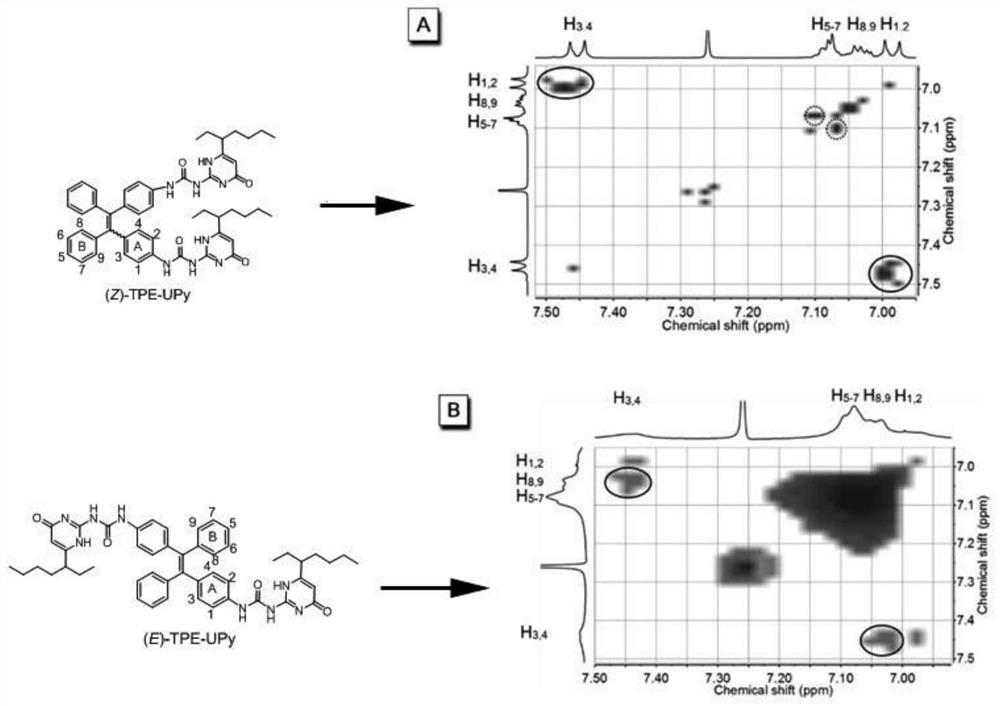
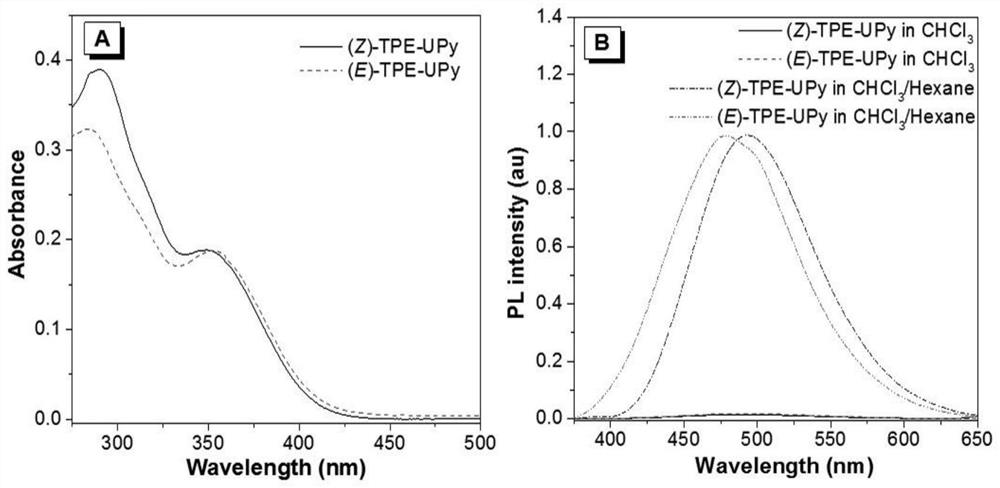
[0045] Preferably in the present invention, R is figure 1 i.e. R is When (Z)-TPE-UPy and (E)-TPE-UPy) synthetic structure diagram, namely:
[0046]
specific Embodiment
[0048] Table 1. Generate five specific examples of (Z)-TPE-UPy and (E)-TPE-UPy
[0049]
[0050] As can be seen from the above table 1, when using Example 1, the yield is the highest, the total yield is as high as 86%, and the (Z)-TPE-UPy and (E)-TPE-UPy yields are relatively average; the excess of compound 2 is beneficial to the product yield rate increase.
[0051] The present invention also provides the raw material compound 1 that can meet the needs of the present invention, i.e. the production method of 1,2-(4-aminobenzene)-1,2-stilbene, the specific steps are as follows: under nitrogen protection, ice bath Conditions: Slowly add 1~12g of titanium tetrachloride (5.3~63.3mmol) to 0.5~5.9g of 4-aminobenzophenone (2.5~30mmol), 0.3~4.2g of zinc powder (5.3~65mmol) in tetrahydrofuran (80mL) Then remove the ice bath from the solution and heat the mixed solution to reflux for 5 h. After the reaction solution drops to room temperature, add saturated potassium carbonate soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com