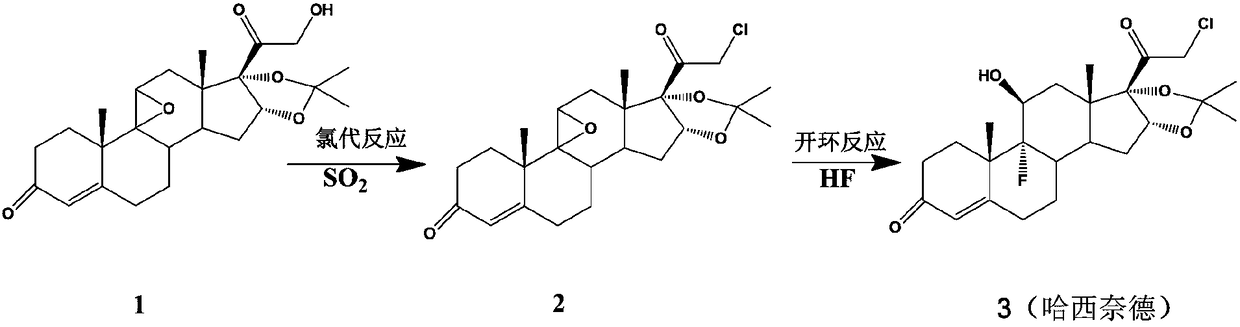
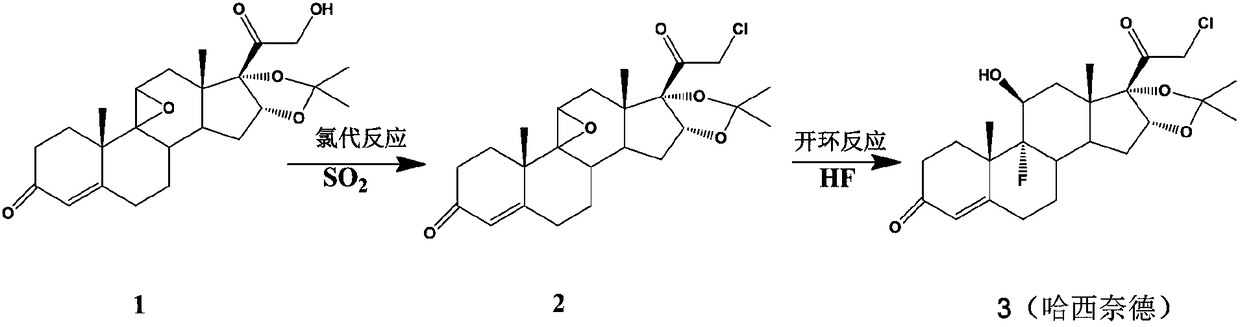
Preparation method of halcinonide
A technology of selecting and compounding, which is applied in the field of medicine, can solve the problems of easy introduction of genotoxic impurities, increased risk of genetic impurities, and low reaction yield, so as to reduce the pressure of environmental protection treatment, control side reactions, increase reaction yield and quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 Chlorination reaction
Embodiment 1-1
[0024] 7.0 g of compound 1 (0.017 mol) was dissolved in 34 ml of pyridine, nitrogen was blown in, and the temperature was lowered to 5°C. Add 2.5g chlorosuccinimide (0.018mol), add dropwise SO 2 / pyridine (15ml, 20%). After reacting for 1.5 h, the reaction solution was diluted into 500 ml of 0° C. water, stirred for 1 h, filtered, and dried to obtain compound 2 (7.2 g, molar yield 97.6%, HPCL purity 98.6%).
Embodiment 1-2
[0028] 14.0 g (0.034 mol) of compound 1 was dissolved in 80 ml of dichloromethane, blown with nitrogen, and cooled to -8°C. Add 15ml of triethylamine, pass through SO 2 (20% by weight of triethylamine absorbed), 2.8 g of acetyl chloride (0.036 mol) was added. After 2 hours of reaction, the reaction solution was diluted to 600ml of 0°C water, stirred for 10 minutes and then separated. The organic phase was distilled off under reduced pressure to obtain compound 2 (13.6g, molar yield 92.2%, HPCL purity 96.4%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com