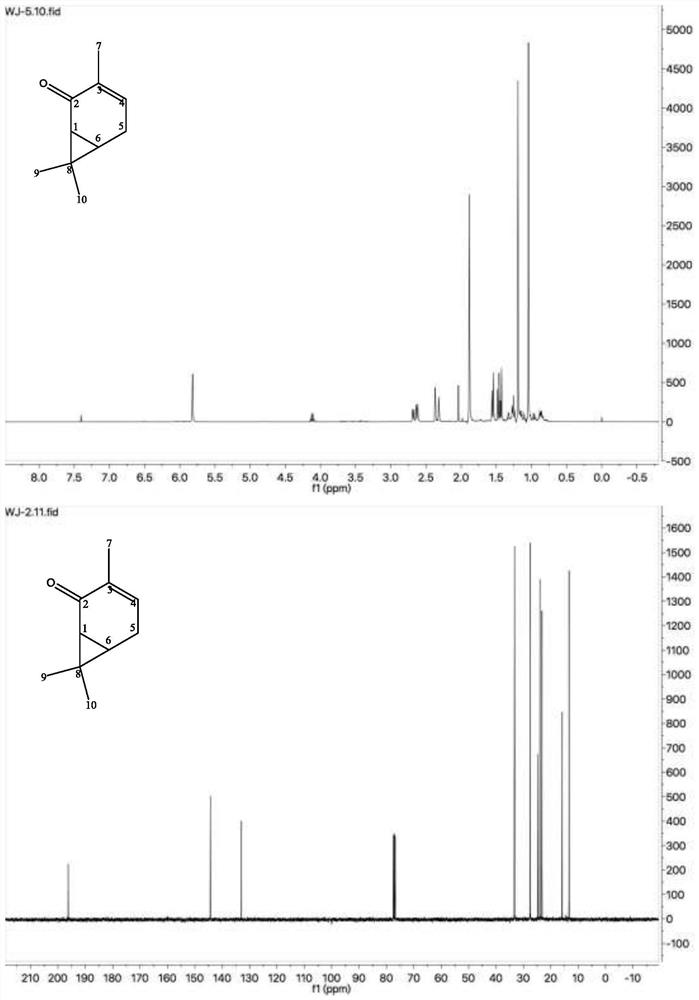
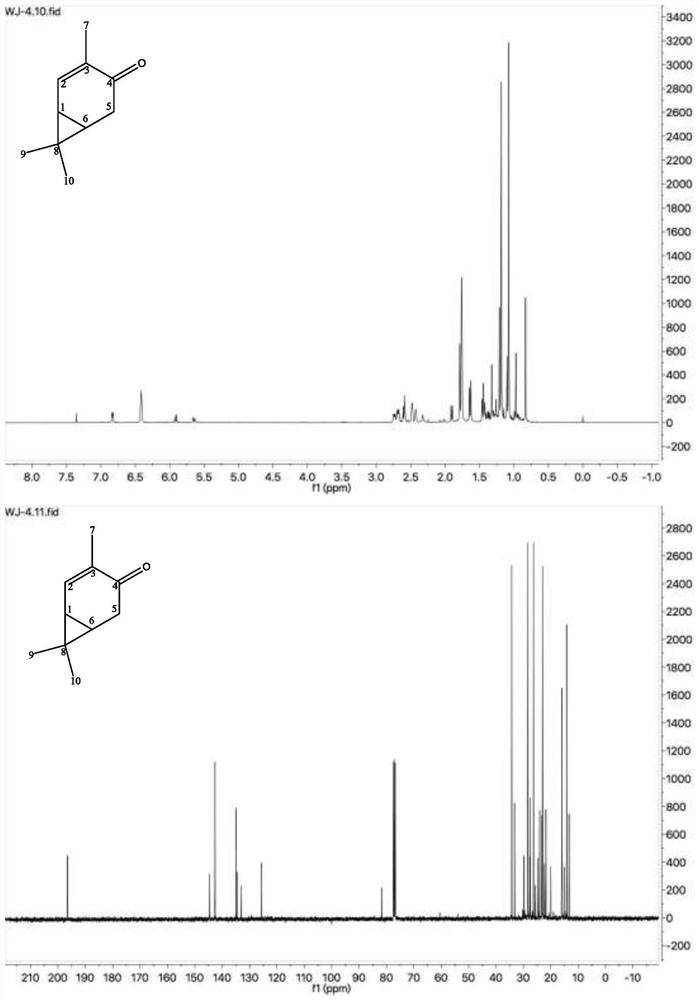
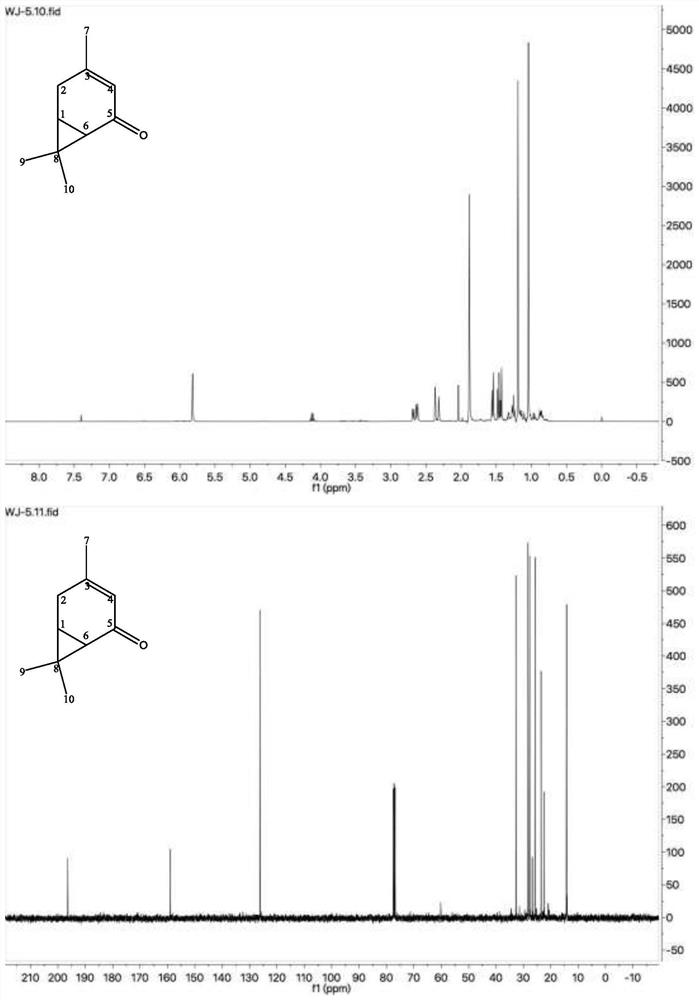
A method for preparing 3-isopropyl-5-cresol and carvacrol from 3-carene
A technology of isopropyl and carvacrol, applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of difficult separation, low selectivity of single product, etc., and achieve high utilization rate of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of product I
[0048] Weigh 5g of anhydrous CrO 3 , configured as a 5% aqueous solution, weigh 5 g of neutral alumina (100 mesh), add it to the above aqueous solution, stir at room temperature for 24 h, filter, and dry at 110 °C for 6 h; weigh 100 g of 3-carene, 3.0 g of CrO 3 -Al 2 O 3 Add to the reaction flask together, pass oxygen, and control the air intake to be 20mL / min, turn on stirring at room temperature to carry out the reaction; take samples every 1h, separate the catalyst by centrifugation, and conduct gas chromatography analysis; after 10h, gas analysis results: 3-carene 41.28%, 2-careone 11.65%, 4-careone 3.31%, 5-careone 35.60%, analysis results after 11h: 3-carene 39.98%, 2-carene 11.33%, 4-carene 3.87%, 5-careone 35.32%, the reaction was stopped, the reaction solution was centrifuged, and the catalyst was recovered.
Embodiment 2
[0049] Example 2 Preparation of product I
[0050] The catalyst was recycled for the third time, and other process conditions were the same as those in Example 1. The results of gas phase analysis after 10 hours: 3-carene 46.62%, 2-carene 10.83%, 4-carene 2.80%, 5-carene 31.29%.
Embodiment 3
[0051] Example 3 Preparation of product I
[0052] The amount of catalyst added was 5.0 g, and the rest of the process conditions were the same as those of Example 1. The results of gas phase analysis after 10 hours: 39.41% of 3-carene, 11.83% of 2-carene, 3.83% of 4-carene, and 35.03% of 5-carene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com