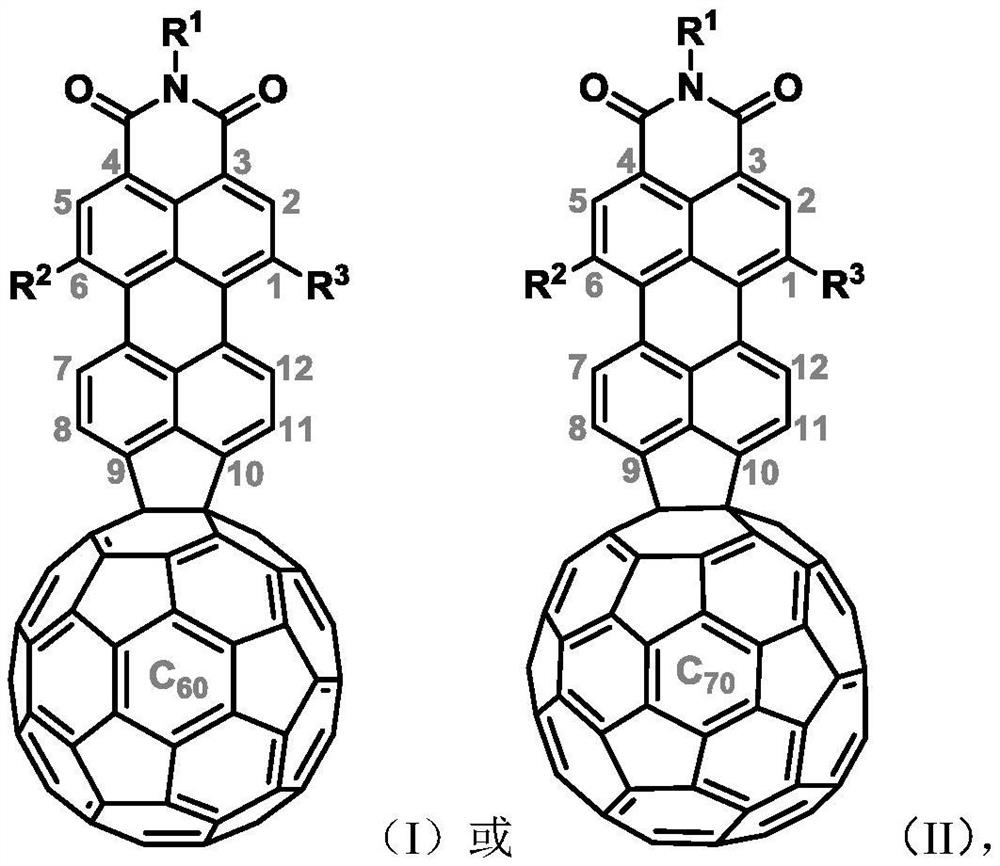
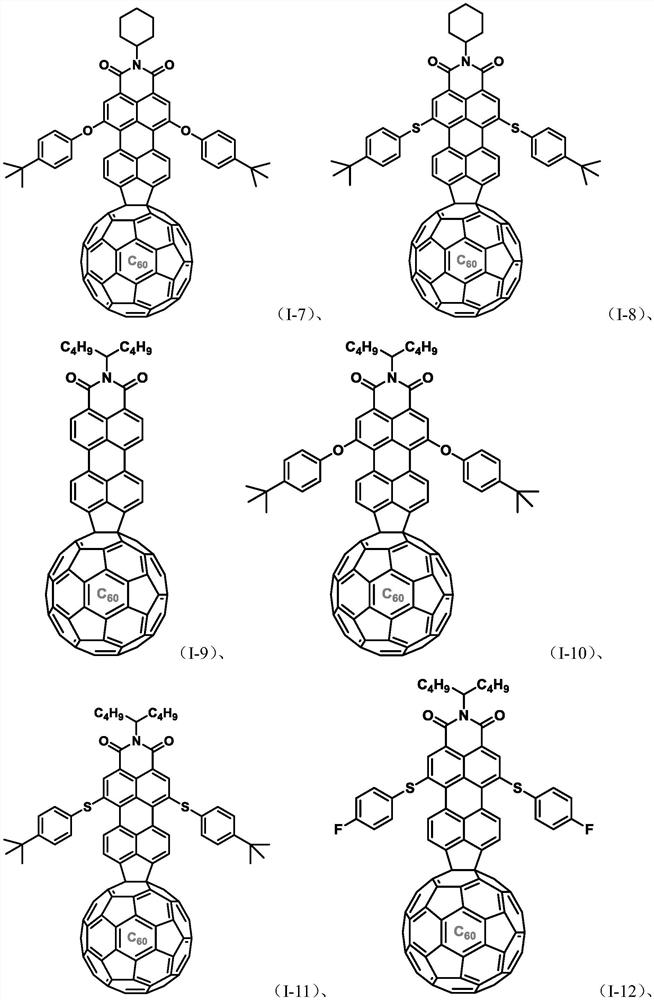
Perylene monoimide peri-fused fullerene derivatives and its preparation method and application
A technology of fullerenes and compounds, applied in the field of organic chemical synthesis, can solve problems such as weak absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
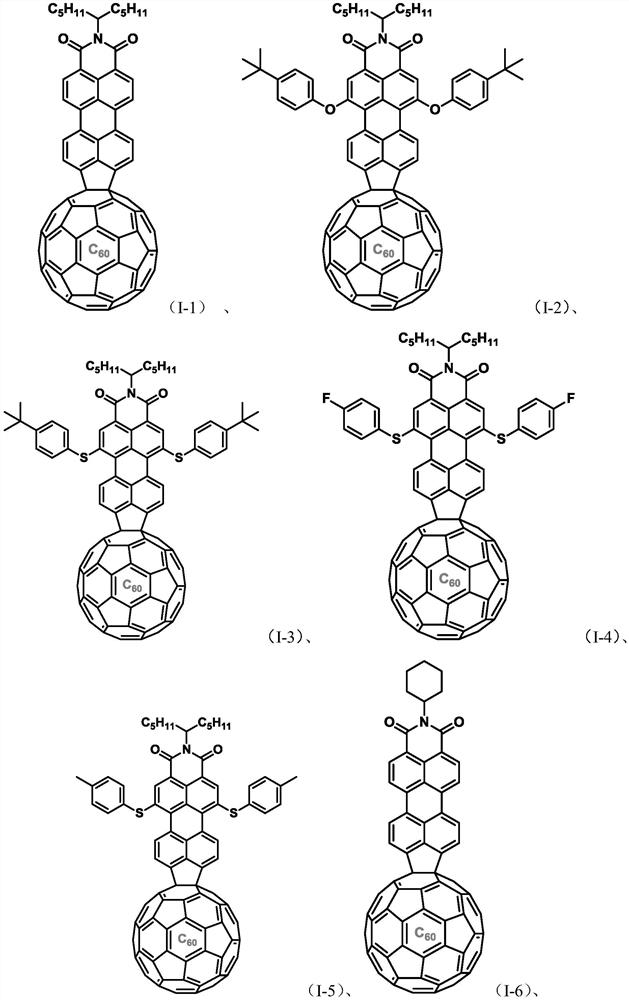
[0101] The preparation of embodiment 1 formula (I-1) compound
[0102] Under nitrogen protection, 9-bromoperylene monoimide (154mg, 0.278mmol), fullerene C60 (200mg, 0.278mmol), palladium acetate, (4mg, 0.017mmol), tricyclohexylphosphine tetrafluoroborate (13mg, 0.034mmol), anhydrous potassium carbonate (153mg, 1.112mmol) and 8mL 1-methylnaphthalene, heated to 200°C, reacted for 3h, cooled After reaching room temperature, the reaction solution was poured into methanol, and the precipitate was collected by suction filtration, washed with methanol, dried, and purified on a silica gel column, using carbon disulfide as a developing solvent to obtain 159 mg of the compound of formula (I-1), with a yield of 48%.
[0103]
[0104] Characterization data of the compound of formula (I-1): 1 H NMR (500MHz, CDCl 2 CDCl 2 ,373K):δ=8.69-8.68(d,J=7.8Hz,4H),8.53-8.52(d,J=8.0Hz,2H),8.32-8.30(d,J=7.6Hz,2H),5.25- 5.19(m,1H),2.33-2.26(m,2H),2.00-1.94(m,2H),1.45-1.31(m,12H),0.92-0.89(m,6H)....
Embodiment 2
[0105] The preparation of embodiment 2 formula (I-2) compound
[0106] Under nitrogen protection, add 1,6-(4-tert-butyl)phenoxy 9-bromoperylene monoimide (237mg, 0.278mmol) represented by formula (III-2) into a sealed tube of 100mL , fullerene C60 (200mg, 0.278mmol), palladium acetate (4mg, 0.017mmol), tricyclohexylphosphine tetrafluoroborate (13mg, 0.034mmol), anhydrous potassium carbonate (153mg, 1.112mmol) and 8mL 1 -Methylnaphthalene, heated to 200°C, reacted for 3h, cooled to room temperature, the reaction solution was poured into methanol, the precipitate was collected by suction filtration, washed with methanol, dried, purified by silica gel column, and carbon disulfide was used as developing agent to obtain the formula (I- 2) Compound 178 mg, yield: 43%.
[0107]
[0108] Characterization data of the compound of formula (I-2): 1 H NMR (500MHz, CDCl 2 CDCl 2 ,373K):δ=9.68-9.66(d,J=8.0Hz,2H),8.37(s,2H),8.28-8.27(d,J=8.0Hz,2H),7.49-7.47(d,J=8.6 Hz,4H),7.20-7.18(d,...
Embodiment 3
[0109] The preparation of embodiment 3 formula (I-3) compound
[0110] Under nitrogen protection, add 1,6-(4-tert-butyl)phenylsulfanyl 9-bromoperylene monoimide (245mg, 0.278mmol) represented by formula (III-3) into a sealed tube of 100mL , fullerene C60 (200mg, 0.278mmol), palladium acetate (4mg, 0.017mmol), tricyclohexylphosphine tetrafluoroborate (13mg, 0.034mmol), anhydrous potassium carbonate (153mg, 1.112mmol) and 8mL 1 -Methylnaphthalene, heated to 200°C, reacted for 3h, cooled to room temperature, the reaction solution was poured into methanol, the precipitate was collected by suction filtration, washed with methanol, dried, purified by silica gel column, and carbon disulfide was used as developing agent to obtain the formula (I- 3) Compound 148 mg, yield: 35%.
[0111]
[0112] Characterization data of the compound of formula (I-3): 1 H NMR (500MHz, CDCl 2 CDCl 2 ,373K):δ=9.34-9.33(d,J=7.7Hz,2H),8.64(s,2H),8.43-8.41(d,J=7.8Hz,2H),7.43-7.39(m,8H), 5.09-5.05(m,1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com