Preparation method of dabigatran intermediate
A compound and reaction technology, applied in new preparation fields, can solve problems such as incomplete reaction of raw materials, high operation risk, and low product yield, and achieve the effect of short steps, mild conditions, and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
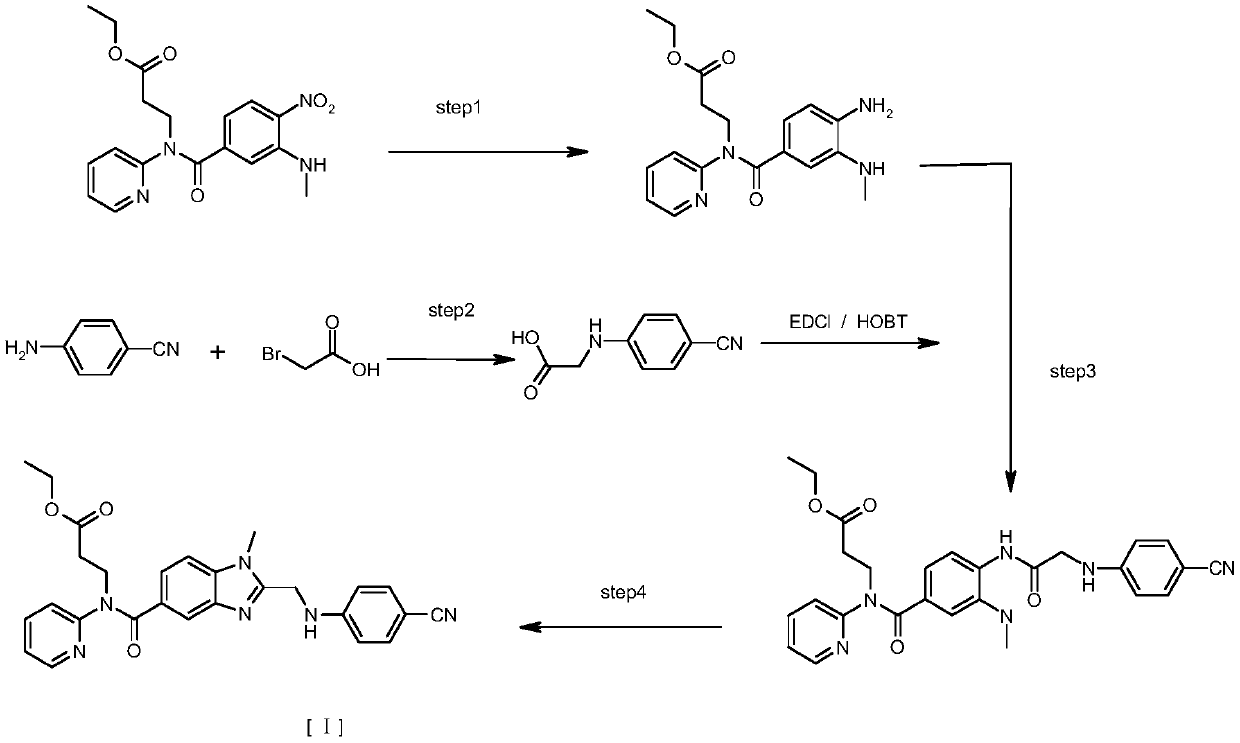
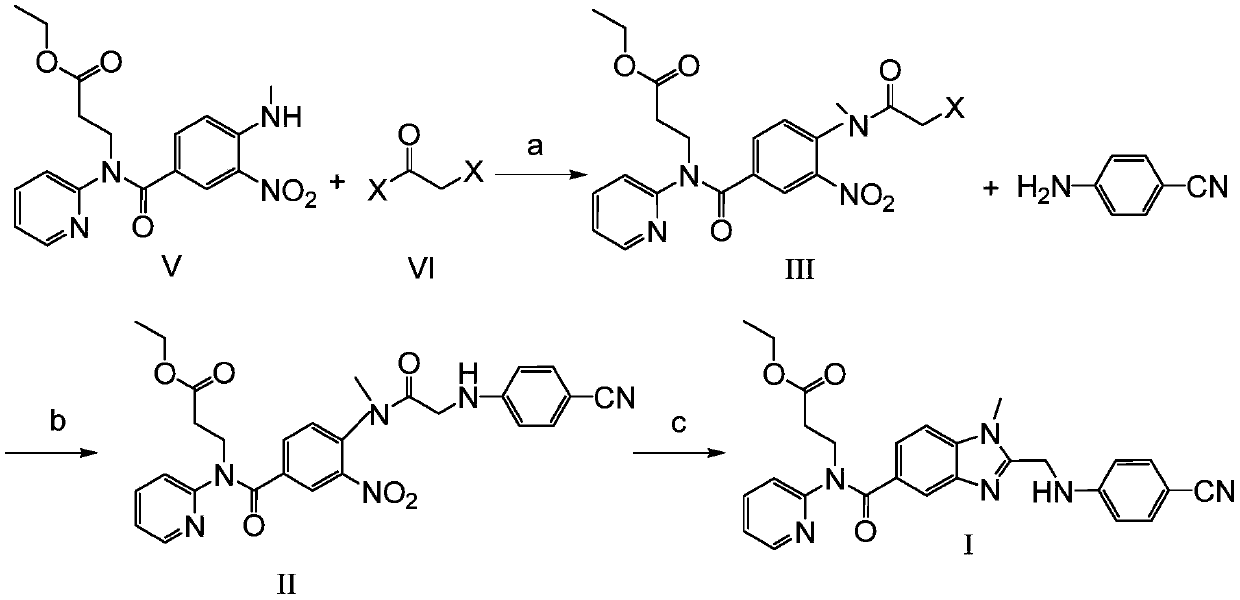
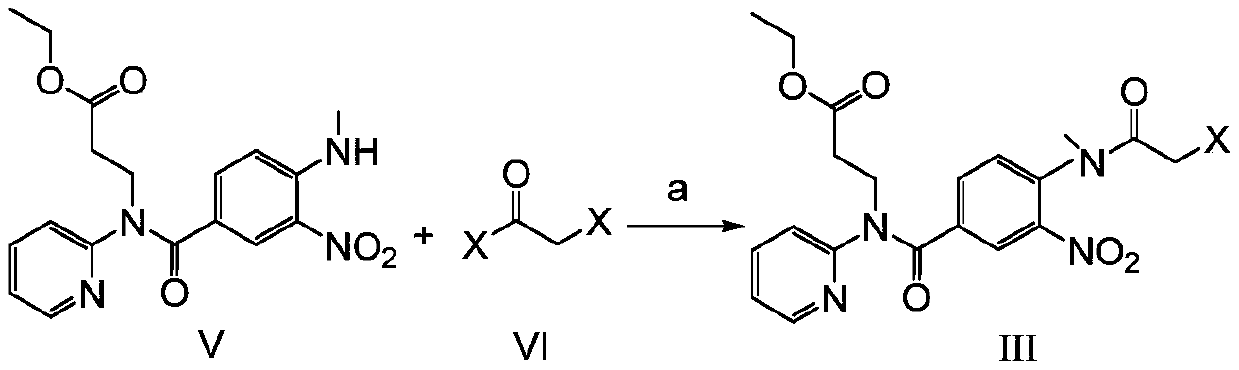
[0032] Preparation of Embodiment 1 Formula III Compound (-X=-Cl)
[0033] Dissolve 37.2g of the compound of formula V in 200ml of dichloromethane, add 15g of triethylamine, control the temperature in an ice-water bath at 0-5 degrees, slowly add 13.8g of chloroacetyl chloride dropwise, and drop it in about 20 minutes. After the drop is completed, remove the ice bath , naturally warming up to room temperature and stirring for 3 to 4 hours. After the reaction, 100ml of water was added, and the layers were separated after stirring. The aqueous layer was extracted twice with 100ml of dichloromethane*2. Dry with 8 g of anhydrous sodium sulfate, filter with suction, concentrate the filtrate to dryness under reduced pressure, and recrystallize the residue with 115 ml of ethyl acetate to obtain 40.2 g of the product.
Embodiment 2
[0034] The preparation of embodiment 2 formula II compound
[0035] Dissolve 22.4 g of the compound of formula III (-X=-Cl) prepared in Example 1 in 120 ml of acetonitrile, add 6.3 g of p-aminobenzonitrile and 10 g of potassium carbonate, and slowly raise the temperature to about 35 degrees under stirring and react for 5 hours Left and right, TLC showed that after the reaction was completed, the acetonitrile was concentrated under reduced pressure to dryness, 120ml of water was added, extracted with 150ml of ethyl acetate*2, the organic layers were combined, washed with 50ml of saturated saline, dried with 10g of anhydrous sodium sulfate, filtered with suction, and the filtrate was reduced Concentrate to dryness under pressure, add 70ml of ethanol to the residue, heat, cool and crystallize, filter and dry to obtain 23.8g of the product.
Embodiment 3
[0036] The preparation of embodiment 3 formula I compound
[0037] Put 20 g of the compound of formula II prepared in Example 2 and 200 ml of ethyl acetate into a hydrogenation tank, stir and dissolve, add 0.4 g of 5% palladium hydroxide, add 1 ml of glacial acetic acid, replace N2 three times with hydrogen, and then pass in hydrogen Control the pressure around 1kg / cm2 to react for 4-6 hours. After the reaction was completed, hydrogen was replaced by nitrogen, the material was discharged, suction filtered, the filtrate was concentrated to dryness under reduced pressure, 95 ml of ethanol was added to the residue, heated to dissolve, and then cooled to crystallize to obtain 14.9 g of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com