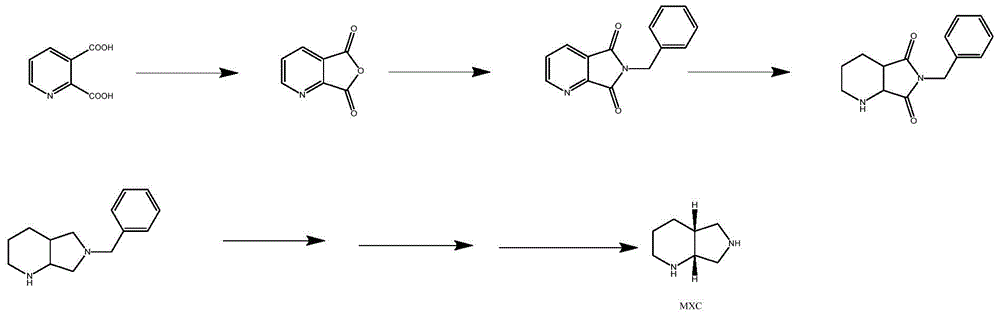
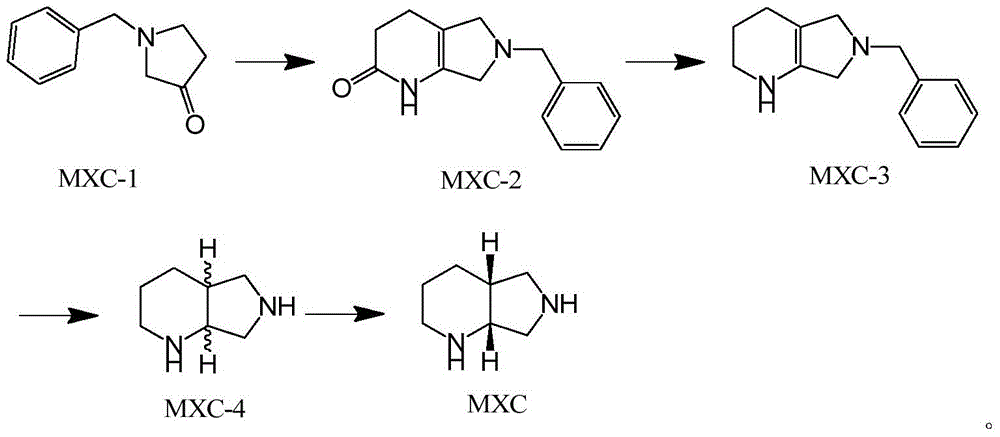
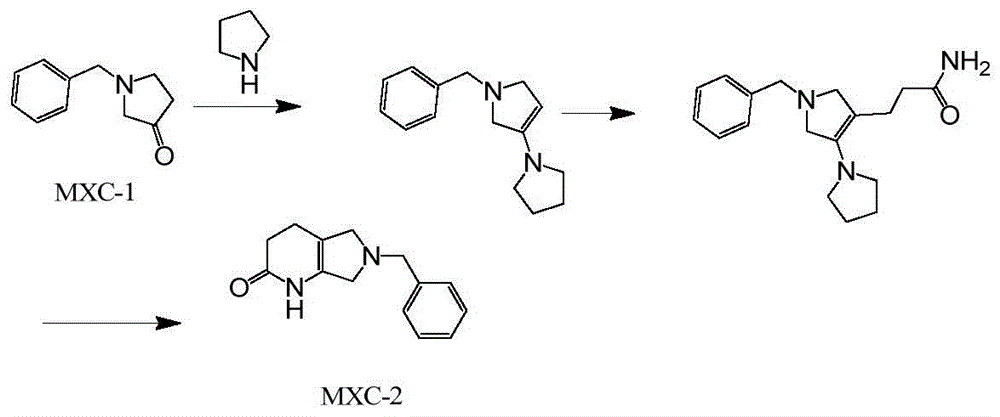
The preparation method of moxifloxacin intermediate
A technology of moxifloxacin and intermediates, applied in new preparation fields, can solve the problems of high price, high cost, complicated raw material preparation process, etc., and achieve the effect of short steps and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of MXC-2
[0024] Put 75g of compound MXC-1175g and 85g tetrahydropyrrole into the bottle, add 700ml of toluene and 10g of p-toluenesulfonic acid into the bottle, heat to reflux to separate water, TLC shows that the reaction is stopped after the reaction is completed, and the toluene is concentrated under reduced pressure to dryness. Add 200g of acrylamide, continue heating to 130-135°C and stir to react for 8-10 hours. After the reaction, cool down to 70°C, add 700ml of ethyl acetate, stir and cool down to crystallize, filter and dry to obtain about 152g of MXC-2, HPLC purity 98.6 %
Embodiment 2
[0025] Embodiment 2 MXC-3 ester preparation
[0026] Put 120ml of THF and 6g of sodium borohydride into the bottle, lower the temperature to below -5°C, slowly add 22g of boron trifluoride tetrahydrofuran dropwise, and drop it in about 30 minutes. After dropping, keep warm for 30 minutes below 10 degrees, slowly add 23g of compound MXC-2 dropwise, after dropping, stir at room temperature for 12-15 hours, stop the reaction, concentrate ethanol to dryness under reduced pressure, add 100ml of water to the residue, concentrate under reduced pressure and recover THF Extract with dichloromethane, wash the organic layer with 50ml of saturated brine, add 10g of anhydrous sodium sulfate to the organic layer, dry it with suction and filter, concentrate the filtrate to dryness under reduced pressure, then add 20.3g of MXC-3, which is directly put into the next step without purification.
Embodiment 3
[0027] Embodiment 3 MXC-4 ester preparation
[0028] Put 20g of the compound MXC-3 and 60 ethanol into the kettle, add 0.4g of 10% palladium on carbon, pass in hydrogen after N2 replacement, control the hydrogen pressure of 0.3-0.5MPa, heat to 40 degrees to react, after the end of the central control reaction, After N2 replacement, pressure filtration was performed, and the filtrate was concentrated to dryness under reduced pressure to obtain 11.1 g of MXC-4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com