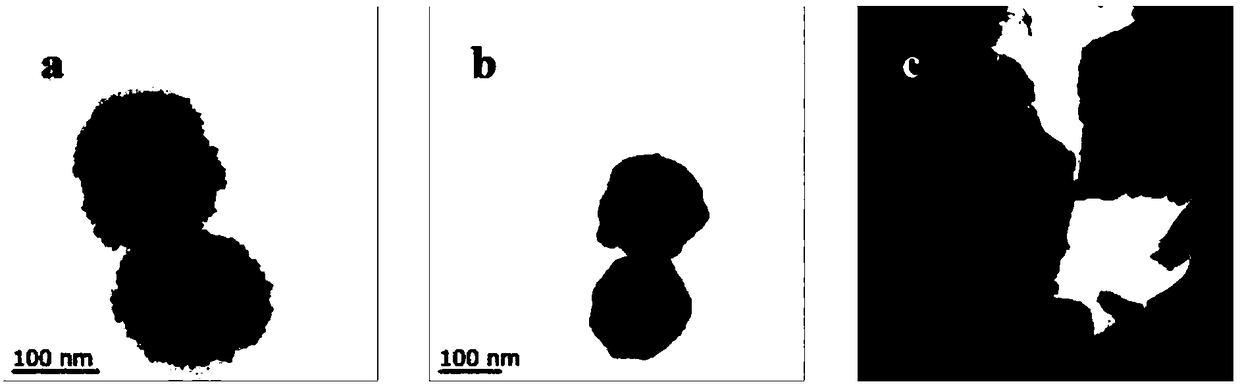
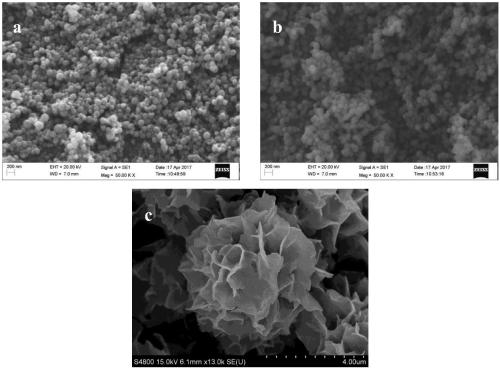
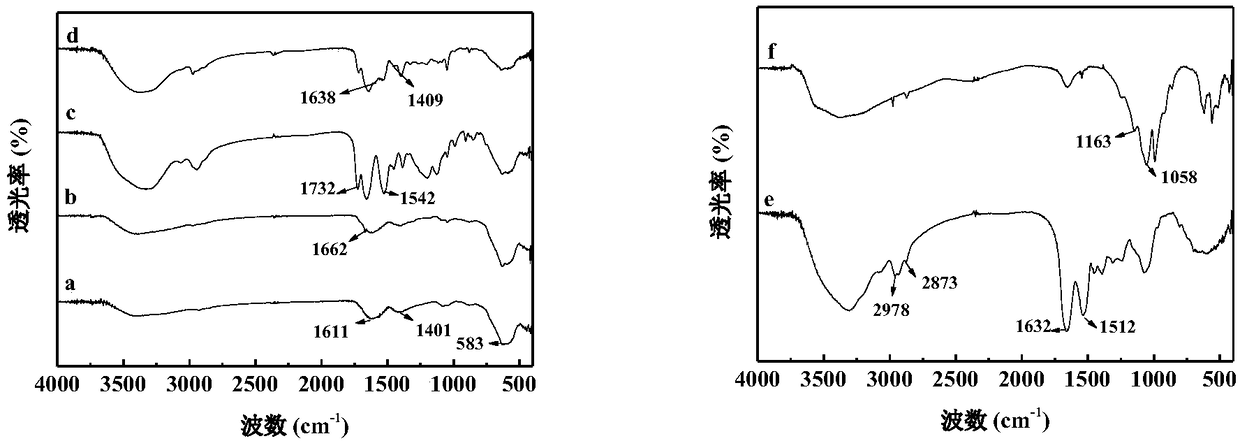
Horseradish peroxidase magnetic nano flower and preparation method and application thereof
A horseradish peroxidase and magnetic nanotechnology, applied in the field of nanomaterials, can solve the problems of inability to rapidly separate the immobilized enzyme, reduce the efficiency of reuse, etc., and achieve the improvement of activity and catalytic activity, improvement of reuse ability, and stability. Sexual improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: Preparation of magnetic composite microspheres
[0074] (1) Ferric oxide nanoparticles (Fe 3 o 4 )Synthesis
[0075] It was prepared by an improved hydrothermal method. 1.050 g of FeCl 3 ·6H 2 O, 3.454 g of NH 4 AC and 0.3 g of trisodium citrate dihydrate were dissolved in a single-necked flask (250 ml) filled with 60 mL of ethylene glycol, 80 o The oil bath of C was heated and stirred for 1 h to obtain a black homogeneous system, which was then transferred to a polytetrafluoroethylene-lined stainless steel autoclave (100 ml), and placed in a 180 o C oven reacted for 16 h, cooled to room temperature, magnetically separated the black product, washed several times with absolute ethanol until the supernatant had no color, and placed at 30 o C dried in a vacuum oven for 24 h.
[0076] (2) Polyacrylic acid modified ferric oxide (Fe 3 o 4 @MPS) Synthesis of microspheres
[0077] Weigh 0.1 g of Fe 3 o 4 Add 40 mL of ethanol, 10 mL of water and 1.5 mL...
Embodiment 2
[0084] Embodiment 2: Preparation of magnetic composite microspheres
[0085] (1) Ferric oxide nanoparticles (Fe 3 o 4 )Synthesis
[0086] It was prepared by an improved hydrothermal method. 1.650 g of FeCl 3 ·6H 2 O, 4.254 g of NH 4 AC and 0.5 g of trisodium citrate dihydrate were dissolved in a single-necked flask (250 ml) filled with 80 mL of ethylene glycol, 120 o The oil bath of C was heated and stirred for 1 h to obtain a black homogeneous system, which was then transferred to a polytetrafluoroethylene-lined stainless steel autoclave (100 ml), and placed in a 220 o C oven reacted for 16 h, cooled to room temperature, magnetically separated the black product, washed several times with absolute ethanol until the supernatant had no color, and placed at 30 o C dried in a vacuum oven for 24 h.
[0087] (2) Polyacrylic acid modified ferric oxide (Fe 3 o 4 @MPS) Synthesis of microspheres
[0088] Weigh 0.5 g of Fe 3 o 4 Add 40 mL of ethanol, 10 mL of water and 1.5 m...
Embodiment 3
[0095] Embodiment 3: Preparation of magnetic composite microspheres
[0096] (1) Ferric oxide nanoparticles (Fe 3 o 4 )Synthesis
[0097] It was prepared by an improved hydrothermal method. 1.350 g of FeCl 3 ·6H 2 O, 3.854 g of NH 4 AC and 0.4 g of trisodium citrate dihydrate were dissolved in a one-necked flask (250 ml) containing 70 mL of ethylene glycol, heated in an oil bath at 100 °C and stirred for 1 h to obtain a black homogeneous system, which was then transferred to polytetrafluoroethylene Vinyl fluoride-lined stainless steel autoclave (100 ml), placed in an oven at 200 °C for 16 h, cooled to room temperature, magnetically separated the black product, washed several times with absolute ethanol until the supernatant had no color, and placed in 30 o C dried in a vacuum oven for 24 h.
[0098] (2) Polyacrylic acid modified ferric oxide (Fe 3 o 4 @MPS) Synthesis of microspheres
[0099] Weigh a certain amount of 0.3 g of Fe 3 o 4 Add 40 mL of ethanol, 10 mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Saturation magnetic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com