Use of L-Plastin gene
A gene and drug technology, applied in the field of screening anti-allergic drugs and inhibitor screening for type I hypersensitivity targeted therapy, can solve the problems of ineffective effect, high cost, and long treatment course
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Constructing a plasmid capable of editing the target gene and transfecting it into cells so that it can function in the cell, and then screening out the cell line with L-Plastin knockout for subsequent experiments
[0050] (1) Plasmid construction
[0051] A. Design and synthesis of gRNA
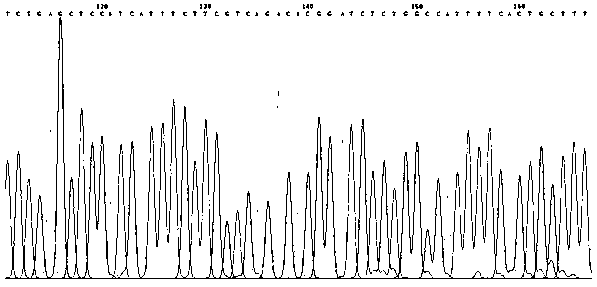
[0052] To design a pair of gDNA of about 20bp, we can design it through the following online tools: CRISPRDesign of MIT: http: / / crispr.mit.edu / ; design the gRNA sequence targeting the gene encoding L-Plastin, as shown in the following table Show:
[0053]
[0054] Note: The lowercase part is the nucleic acid sequence connected to the plasmid;
[0055] B. Cloning sgRNA into pSpCas9(BB) plasmid
[0056] Dissolve each gRNA to a final concentration of 100 μM, and phosphorylate and anneal the sgRNA according to the following recipe;
[0057] Place the above mixture in a PCR machine, and use the following program to phosphorylate and anneal sgRNA: 37°C for 30 minutes...
Embodiment 2
[0093] Example 2: Mast cell sensitizer C48 / 80 can induce mast cell activation and degranulation, and the degree of degranulation has a certain correlation with the ability of mast cells to accept C48 / 80 sensitization signals
[0094] In order to verify the effect of L-Plastin on mast cells accepting C48 / 80 signal, we used CCK-8 assay to detect the cell proliferation inhibition rate of P815 WT and L-Plastin KO cells induced by C48 / 80 (10 μg / mL), Specific steps are as follows:
[0095] (1) Culture and passage of mast cell lines P815 WT and L-Plastin KO cells
[0096] A. Cultivation of P815 WT and L-Plastin KO cells: P815 was cultured in high-glucose DMEM medium containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin at 37°C and 5% CO 2 Environment;
[0097] B. Passaging of P815 WT and L-Plastin KO cells: When the cell density reaches 80% in step A, passaging is required. Collect the cell culture solution in the culture flask into a centrifuge tube and rinse wit...
Embodiment 3
[0105] Experimental purpose and method: The degree of degranulation of mast cells directly reflects the condition of the body's allergic reaction. The pre-synthesized granule medium in the cells contains substances such as histamine and β-hexosaminidase, which can act as mast cell degranulation. Particle markers. In order to verify the effect of L-Plastin on mast cell activation and degranulation, this experiment uses toluidine blue staining method, ELISA method and chromogenic method for detection. The specific steps are as follows:
[0106] (1) Culture and passage of mast cell lines P815 WT and L-Plastin KO cells
[0107] A. The culture of P815 WT and L-Plastin KO cells: P815 was cultured in high-glucose DMEM medium, containing 10% fetal bovine serum (FBS), 1% penicillin and streptomycin, cultured at 37°C, 5% CO 2 Environment;
[0108] B. Passaging of P815 WT and L-Plastin KO cells: When the cell density reaches 80% in step A, passaging is required. Collect the cell cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com