Method for preparing fluorescence precise adjustable perovskite nanocrystals by using metal halogenated inorganic salt aqueous solution as anion exchange reagent
An inorganic salt solution, metal halide technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, inorganic chemistry, etc., can solve problems such as difficult to achieve continuous and precise control of the full spectrum, low reactivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 450mg of zinc bromide powder into 0.5mL of water, shake to dissolve, and prepare an aqueous solution of zinc bromide for use. Add 0.5 μL zinc bromide aqueous solution to 2 mL pre-prepared CsPbCl 3 Nanocrystalline (Nano Lett.2015, 15, 3692-3696) toluene solution, fully oscillated under 20 watts of ultrasonic power, after 5 minutes of ultrasonication at room temperature, the anion-exchanged CsPbCl 0.004 Br 2.996 Perovskite nanocrystals. Centrifuge at 8000 rpm for 5 minutes and centrifuge twice in total. The size of the nanocrystals of this sample does not change significantly, and the average size is 14.9nm of CsPbCl 0.004 Br 2.996 Perovskite nanocrystals, the fluorescence color is in the violet region, and the final fluorescence emission peak is at 408 nanometers. Finally, the CsPbCl in the above violet region can be 0.004 Br 2.996 The perovskite nanocrystals were dissolved in toluene to obtain a nanoparticle solution with a concentration of 0.03mol / L.
Embodiment 2
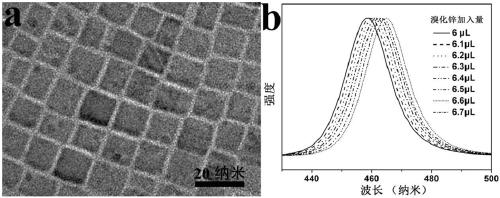
[0017]Add 450mg of zinc bromide powder into 0.5mL of water, shake to dissolve, and prepare an aqueous solution of zinc bromide for use. Add 6 μL, 6.1 μL, 6.2 μL, 6.3 μL, 6.4 μL, 6.5 μL, 6.6 μL, 6.7 μL of zinc bromide aqueous solution to 2 mL of pre-prepared CsPbCl 3 Nanocrystal (Nano Lett.2015, 15, 3692-3696) in toluene solution, fully oscillated under the ultrasonic power of 100 watts, after 30 seconds of ultrasonication at room temperature, the anion-exchanged CsPbCl x Br 3-x Perovskite nanocrystals (0.048≤x≤0.0536). Centrifuge at 8,000 rpm for 5 minutes and centrifuge twice in total. The size of the nanocrystals of multiple samples does not change significantly, and the average size is 15.2nm for CsPbCl x Br 3-x (0.048≤x≤0.0536) perovskite nanocrystals, the fluorescence color is in the blue light region, and the final fluorescence emission peaks are at 458 nm, 459 nm, 460 nm, 461 nm, 462 nm, 463 nm, 464 nm, 465 nm , the fluorescence spectrum realizes the continuous chan...
Embodiment 3
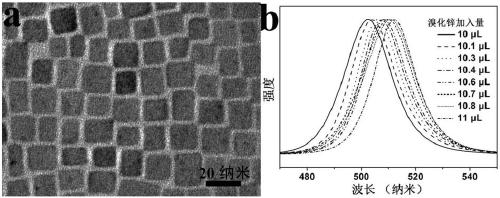
[0019] Add 450mg of zinc bromide powder into 0.5mL of water, shake to dissolve, and prepare an aqueous solution of zinc bromide for use. Add 10 μL, 10.1 μL, 10.3 μL, 10.4 μL, 10.6 μL, 10.7 μL, 10.8 μL, 11 μL of zinc bromide aqueous solution to 2 mL of pre-prepared CsPbCl 3 Nanocrystalline (Nano Lett.2015, 15, 3692-3696) in toluene solution, fully oscillated at 100 watts of ultrasonic power, after 1 minute of ultrasonication at room temperature, the anion-exchanged CsPbCl x Br 3-x Perovskite nanocrystals (0.08≤x≤0.088). Centrifuge at 8,000 rpm for 5 minutes and centrifuge twice in total. The size of the nanocrystals of multiple samples does not change significantly, and the average size of CsPbCl is 15.9 nanometers. x Br 3-x (0.08≤x≤0.088) perovskite nanocrystals, the fluorescence color is in the blue light region, and the final fluorescence emission peaks are at 502 nm, 503 nm, 504 nm, 505 nm, 506 nm, 507 nm, 508 nm, 509 nm . , the fluorescence spectrum realizes the conti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com