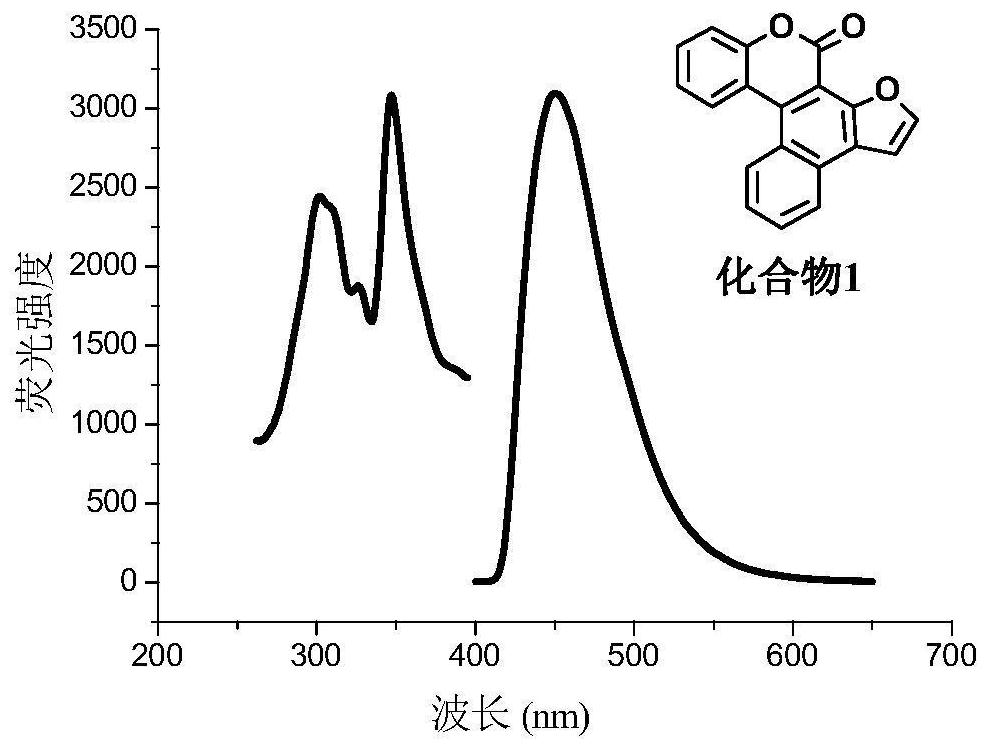
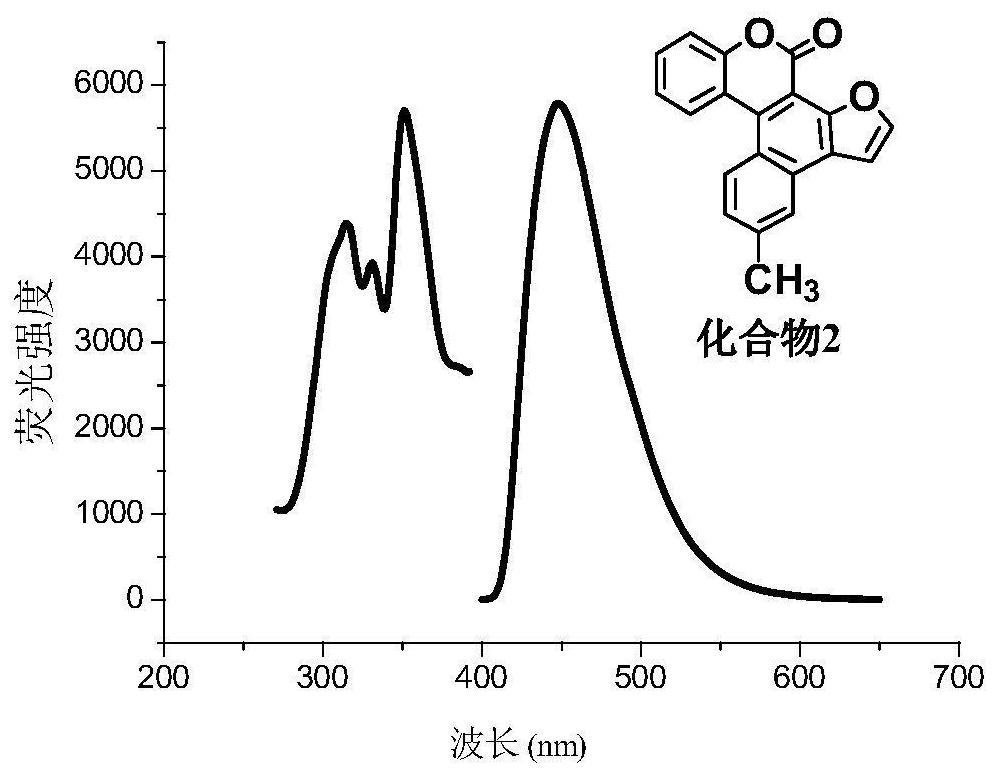
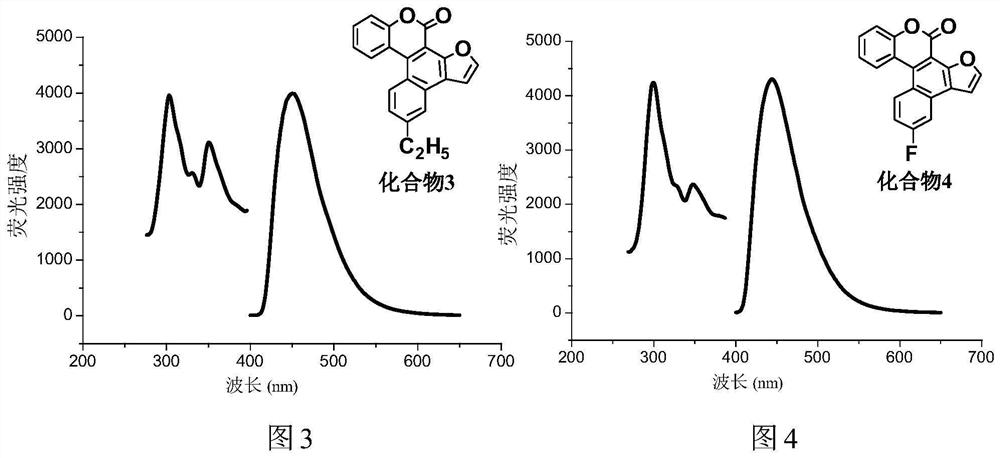
A kind of synthetic method of polybenzo five-membered aromatic heterocyclic coumarin condensed heterocyclic compound
A synthesis method and compound technology, applied in the field of heterocyclic compound synthesis, can solve the problems of high toxicity of reagents, harsh reaction conditions, inconvenient source of raw materials, etc., and achieve the effects of good substrate adaptability and high group tolerance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthetic compound 1~18
[0034] Add 3-(2-furyl)-4-(phenyl)coumarin into the light tube, and use ethanol as solvent to prepare a solution with a concentration of 0.0025mol / L. Under protected conditions, after 2 hours of ultraviolet light irradiation by a 500W high-pressure mercury lamp, the solvent was recovered by distillation under reduced pressure, and it was separated and purified by silica gel column chromatography (petroleum ether:ethyl acetate=50:1) to obtain the compound 1 pure product.
[0035] In this embodiment, with equimolar 3-(2-furyl)-4-(4-methylphenyl) coumarin, 3-(2-furyl)-4-(4-ethylphenyl) Coumarin, 3-(2-furyl)-4-(4-fluorophenyl)coumarin, 3-(2-furyl)-4-(4-chlorophenyl)coumarin, 3-(2-furyl)-4-(4-trifluoromethylphenyl)coumarin, 3-(2-benzofuryl)-4-(4-methylphenyl)coumarin, 3-(2-benzofuryl)-4-(4-methoxyphenyl)coumarin, 3-(2-benzofuryl)-4-(4-fluoro)coumarin, 3 -(2-thienyl)-4-(phenyl)coumarin, 3-(2-thienyl)-4-(4-methylphenyl)coumarin, 3-(2-thienyl)- 4-(...
Embodiment 2
[0065] In this example, an equal volume of methanol was used to replace the ethanol in Example 1, and other steps were the same as in Example 1 to obtain compounds 1-18, and the yields of each compound are shown in Table 4.
[0066] Table 4 is the productive rate of compound 1~18 when methanol is solvent
[0067] Compound 1 Compound 2 Compound 3 Compound 4 Compound 5 Compound 6 50% 55% 53% 36% 31% 43% Compound 7 Compound 8 Compound 9 Compound 10 Compound 11 Compound 12 55% 68% 56% 54% 62% 71% Compound 13 Compound 14 Compound 15 Compound 16 Compound 17 Compound 18 36% 29% 57% 40% 65% 45%
Embodiment 3
[0069] In this example, an equal volume of acetonitrile was used to replace the ethanol in Example 1, and other steps were the same as in Example 1 to obtain compounds 1-18. The yields of each compound are shown in Table 5.
[0070] Table 5 is the productive rate of compound 1~18 when acetonitrile is solvent
[0071] Compound 1 Compound 2 Compound 3 Compound 4 Compound 5 Compound 6 40% 44% 43% 31% 29% 33% Compound 7 Compound 8 Compound 9 Compound 10 Compound 11 Compound 12 43% 50% 52% 51% 55% 63% Compound 13 Compound 14 Compound 15 Compound 16 Compound 17 Compound 18 32% 26% 50% 50% 53% 39%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com