A kind of hydration icariin nanoparticle and its preparation method and application
A hydrated icariin and nanoparticle technology, applied in the field of medicine, can solve the problems of difficulty in administration, limitation of clinical application of HICT in vivo research, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The present invention provides the preparation method of the hydrated epimeditin nano-particle described in above-mentioned technical scheme, comprises the following steps:
[0044] mixing icariin hydrate, a stabilizer, an organic solvent and water to obtain a precursor solution;
[0045] The organic solvent in the precursor solution is removed to obtain hydrated icariin nanoparticles.
[0046] The invention mixes hydration icariin, stabilizer, organic solvent and water to obtain a precursor solution. In the present invention, the concentration of icariin hydrate in the precursor solution is preferably 0.01-100 mg / mL, more preferably 0.1-50 mg / mL, and most preferably 1-30 mg / mL. In the present invention, the organic solvent is preferably the first organic solvent, or a mixture of the first organic solvent and the second organic solvent; the first organic solvent preferably includes methanol, ethanol, acetone, dimethylsulfoxide and One or more of N,N-dimethylformamide,...
Embodiment 1
[0063] Dissolve 20mg HICT and 20mg vitamin E-mPEG1000 succinate in 1mL DMSO as the organic phase; add the organic phase dropwise to 10mL water under 1000r / min magnetic stirring, centrifuge at 13000r / min for 20min, and use 10mL deionized water for precipitation Ultrasonic dispersion for 20 min, 2000 bar homogenization 10 times, to obtain hydrated icariin nanoparticles (HICT-NPs).
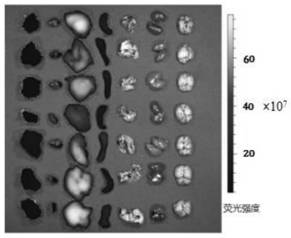
[0064] 1. Characterize the HICT-NPs, as follows:
[0065] (1) Particle size, polydispersity index (PDI), Zeta potential investigation
[0066] A Zetasizernano ZS particle size analyzer was used to measure the particle size distribution and Zeta potential of the HICT-NPs at 25 °C through the principle of dynamic light scattering (DLS), and each sample was measured 3 times in parallel.
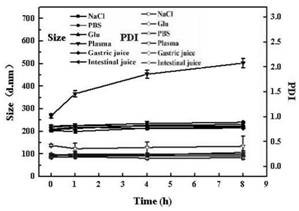
[0067] figure 1 It is the particle size distribution diagram of the HICT-NPs (n=3, mean±SD), the results show that the average particle size is (201.7±1.3) nm, and the PDI is 0.17±0.07, indicating that the particle s...
Embodiment 2
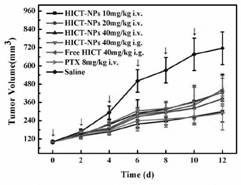
[0158] A series of HICT-NPs were prepared under different conditions, and the particle size, PDI and Zeta potential were investigated, as follows:
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com