Catalyst loaded with alpha-diimine metal complex and application of catalyst to olefin polymerization
A technology of metal complexes and diimine compounds, which is applied in the field of catalysts loaded with α-diimine metal complexes, can solve the problems of high cost, general high temperature resistance of catalysts, complex ligand synthesis steps, etc., and achieve the goal of preparing The effect of low cost, good heat resistance and high temperature catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
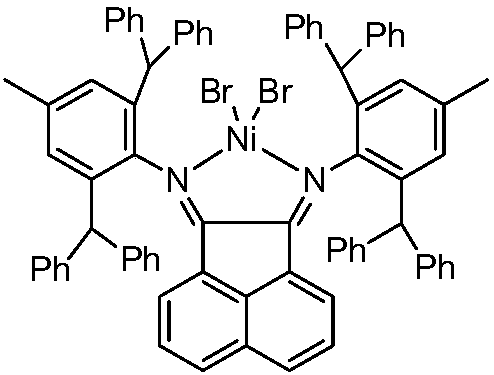
[0037] Synthesis of 5-(4-hydroxymethylphenyl)acenaphthenequinone bis(2,6-diisopropyl)benimide nickel bromide(NiI1)
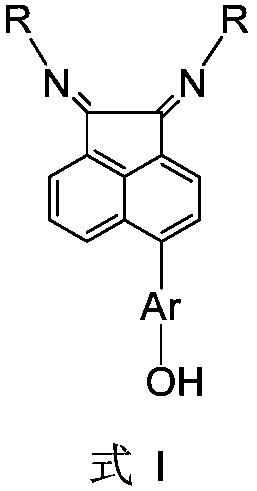
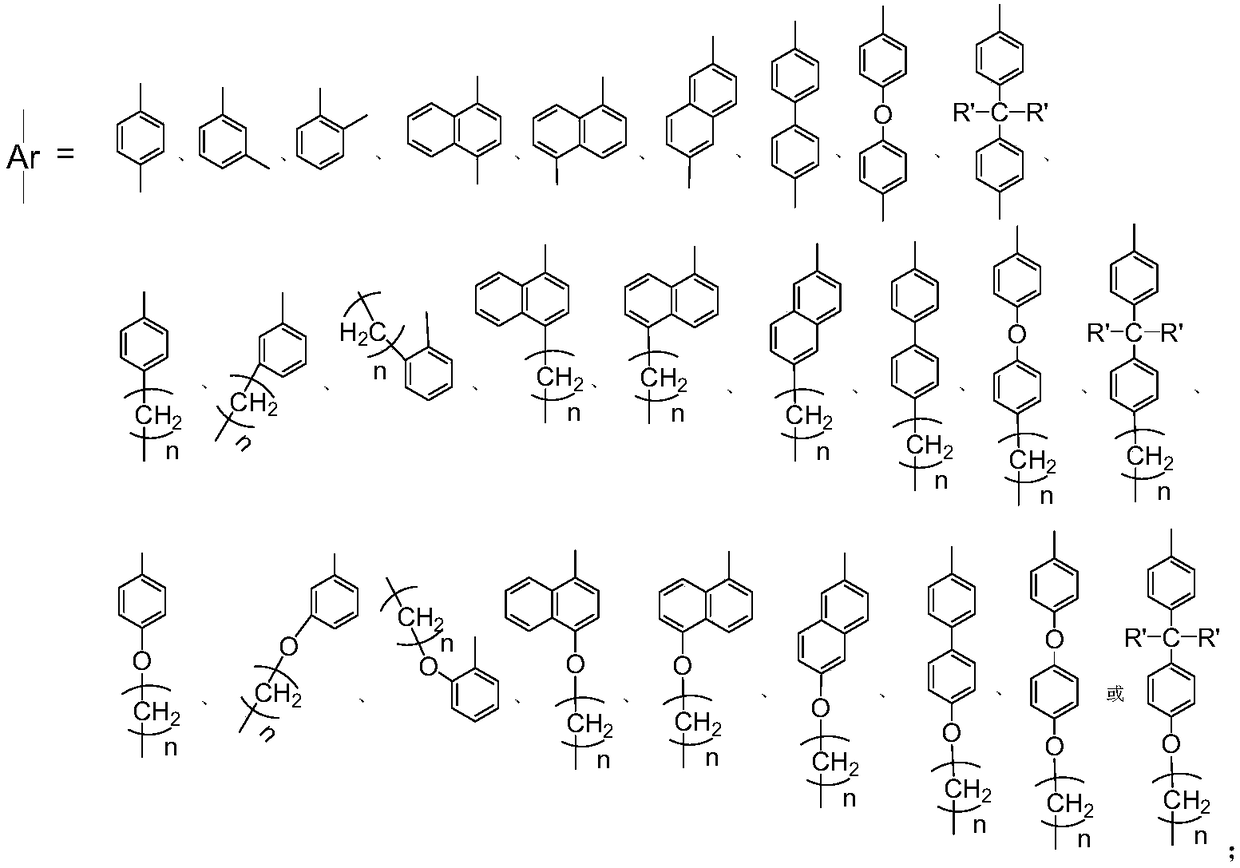
[0038] Ligand 5-(4-hydroxymethylphenyl)acenaphthenequinone bis(2,6-diisopropyl)phenylimine (I1) (i.e. the structure of compound I, wherein Ar=p-methylphenyl, R = (2,6-diisopropyl) phenyl) the preparation route is as follows:
[0039]
[0040] Synthesis of 5-dibromoacenaphthoquinone bis(2,6-diisopropyl)phenylimine a1:
[0041] Add 5-bromoacenaphthenequinone (2.09g, 8mmol), 2,6-diisopropylaniline (3.36g, 19mmol) and 150mL of anhydrous methanol into a 250mL reaction flask, and add 10 drops of anhydrous formic acid , and the mixture was refluxed for 48 hours. The reaction was tracked by chromatography until the raw materials were completely reacted. After the reaction mixture was cooled, a brown-red solid was obtained by suction filtration. The solid was purified by silica gel chromatography to obtain 4.27 g of a yellow solid a1 with a yield of 92%. 1 H NMR (4...
Embodiment 2
[0047] Synthesis of 5-(4-hydroxyphenyl)acenaphthenequinone bis[2,4-dimethyl-6-bis(4-fluorophenyl)methyl]phenylimide nickel bromide (NiI2)
[0048] Ligand 5-(4-hydroxyphenyl)acenaphthenequinone bis[2,4-dimethyl-6-bis(4-fluorophenyl)methyl]phenylimine (I2) (i.e. the structure of compound I, Wherein Ar=p-phenyl, R=[2,4-dimethyl-6-bis(4-fluorophenyl)methyl]phenyl) The preparation route is as follows:
[0049]
[0050] The preparation of compound I2 is the same as that of compound I1 in Example 1, wherein 2,4-dimethyl-6-bis(4-fluorophenyl)methylaniline is used instead of 2,6-diiso Propylaniline, toluene instead of methylene chloride in Example 1, p-toluenesulfonic acid instead of formic acid in Example 1, 4-hydroxybenzopinacol borate instead of 4-hydroxymethyl in Example 1 Phenylboronic acid, bis(dibenzylideneacetone) palladium replaces tetrakis(triphenylphosphine) palladium in embodiment 1, and anhydrous sodium carbonate replaces anhydrous potassium carbonate in embodiment 1. ...
Embodiment 3
[0053] Synthesis of 5-(4-hydroxymethylphenyl)acenaphthenequinone bis[2,6-bis(benzhydryl)-4-methoxy]phenylimide nickel bromide (NiI3)
[0054] Ligand 5-(4-hydroxymethylphenyl) acenaphthoquinone bis[2,6-bis(benzhydryl)-4-methoxyl group] phenylimine (I3) (i.e., the structure of compound I, wherein Ar=p-methylphenyl, R=[2,6-bis(benzhydryl)-4-methoxy]phenyl) The preparation route is as follows:
[0055]
[0056] The preparation of compound I3 is the same as that of compound I1 in Example 1, wherein 2,6-benzhydryl-6-methoxyaniline is used instead of 2,6-diisopropylaniline in Example 1, and toluene is used instead Dichloromethane in Example 1, p-toluenesulfonic acid replaces the formic acid in Example 1, and anhydrous cesium carbonate replaces the anhydrous potassium carbonate in Example 1. The yield of compound I3 was 79%. 1 H NMR (400MHz, CDCl 3): δ7.66(d, J=8.4Hz, 1H), 7.57(d, J=7.9Hz, 2H), 7.41(d, J=7.9Hz, 2H), 7.21-7.12(m, 20H), 6.96 -6.91(m,9H),6.76-6.61(m,17H),6.34(d,J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com