Secondary-stage drug release vaginal administration preparation and preparation method thereof
A technology for vaginal administration and preparation, applied in the field of medicine, can solve the problems of limited administration time, increased production and drug costs for patients, and inability to exercise, etc., to achieve easy carrying storage and administration, increase storage validity period, and inhibit growth and reproduction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] (1) The preparation process of acid source granules and alkali source granules:
[0036] Preparation of acid source granules / alkali source granules (wet method / fluidized bed granulation): Take the prescribed amount of API, filler, acid source / alkali source and disintegrant respectively and pass through a 40-60 mesh sieve for 3 times, crush and remove Polymerized granules; then the binder is formulated into a solution with a mass fraction of 5-10%, and fluidized bed granulation or wet granulation is performed; the prepared granules enter the 0.4-1.2mm aperture screen equipment for processing Granulation, moisture and particle size are detected during the granulation process, and the particle size of the final particle is controlled at 100-500 μm.
[0037] Preparation of acid source granules / alkali source granules (dry granulation): respectively take the prescribed amount of API, filler, acid source / alkali source, disintegrant, and flow aid and pass through a 40-60 mesh s...
Embodiment 1
[0045] The preparation process of a kind of secondary drug release vaginal administration preparation is as follows:
[0046] Prescription 1:
[0047] Each component and its mass ratio in the prescription 1 of table 2
[0048]
[0049]
[0050] (1) Preparation of acid granules: take tinidazole 100g, microcrystalline cellulose 200g, citric acid 100g, sodium carboxymethyl cellulose 20g, mix 40 mesh manual sieves 3 times, and add in the bottom of the fluidized bed; Weigh 20g of hypromellose and add 380mL of water to prepare a binder solution with a mass fraction of 5%, set and adjust the fluidized bed parameters during the preparation process, the inlet air temperature is 55-60°C, and the air volume is 40-50m 3 / h, atomization pressure 0.6-1 bar, feeding peristaltic pump speed 4 rpm, the particle size of the prepared particles is controlled at 100-500 μm, the water content is controlled at <4%, and the acid particles are obtained for use.
[0051] (2) Preparation of alkal...
Embodiment 2
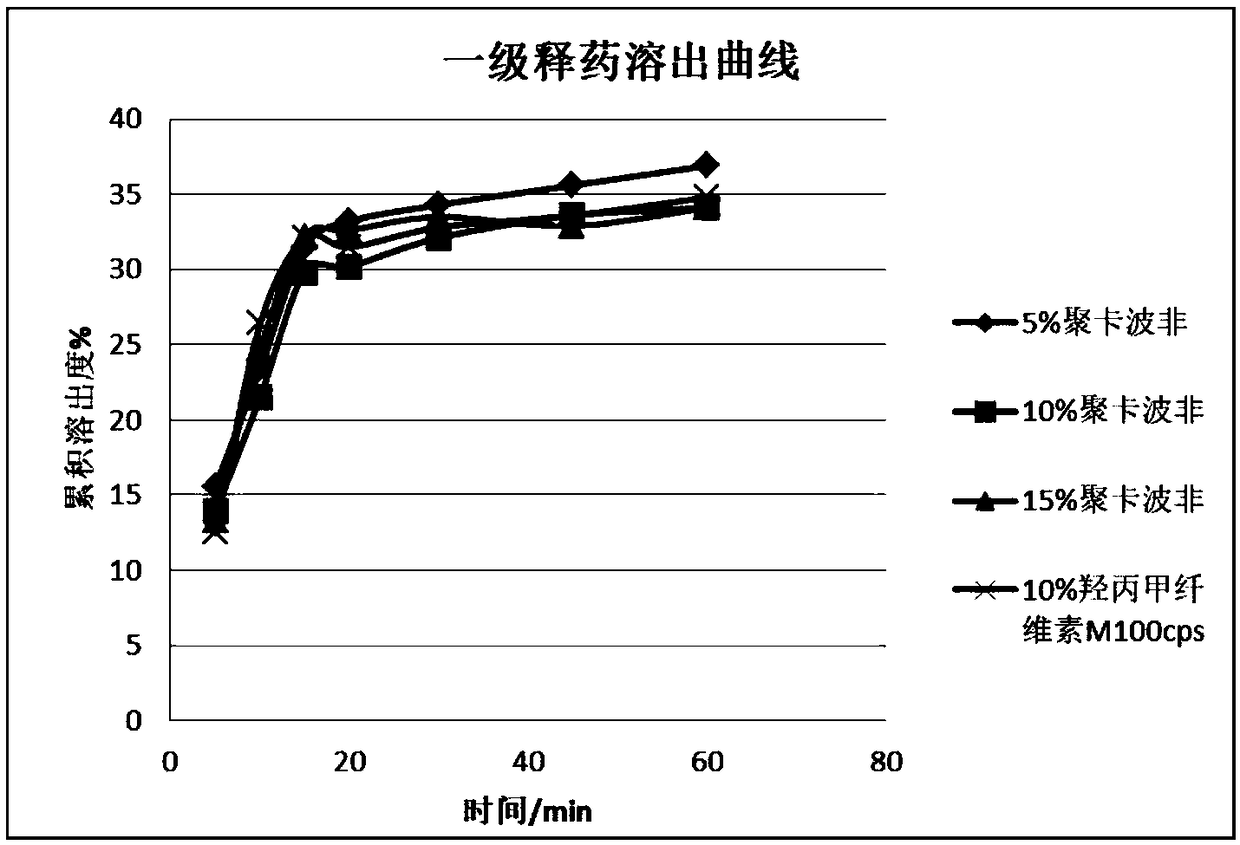
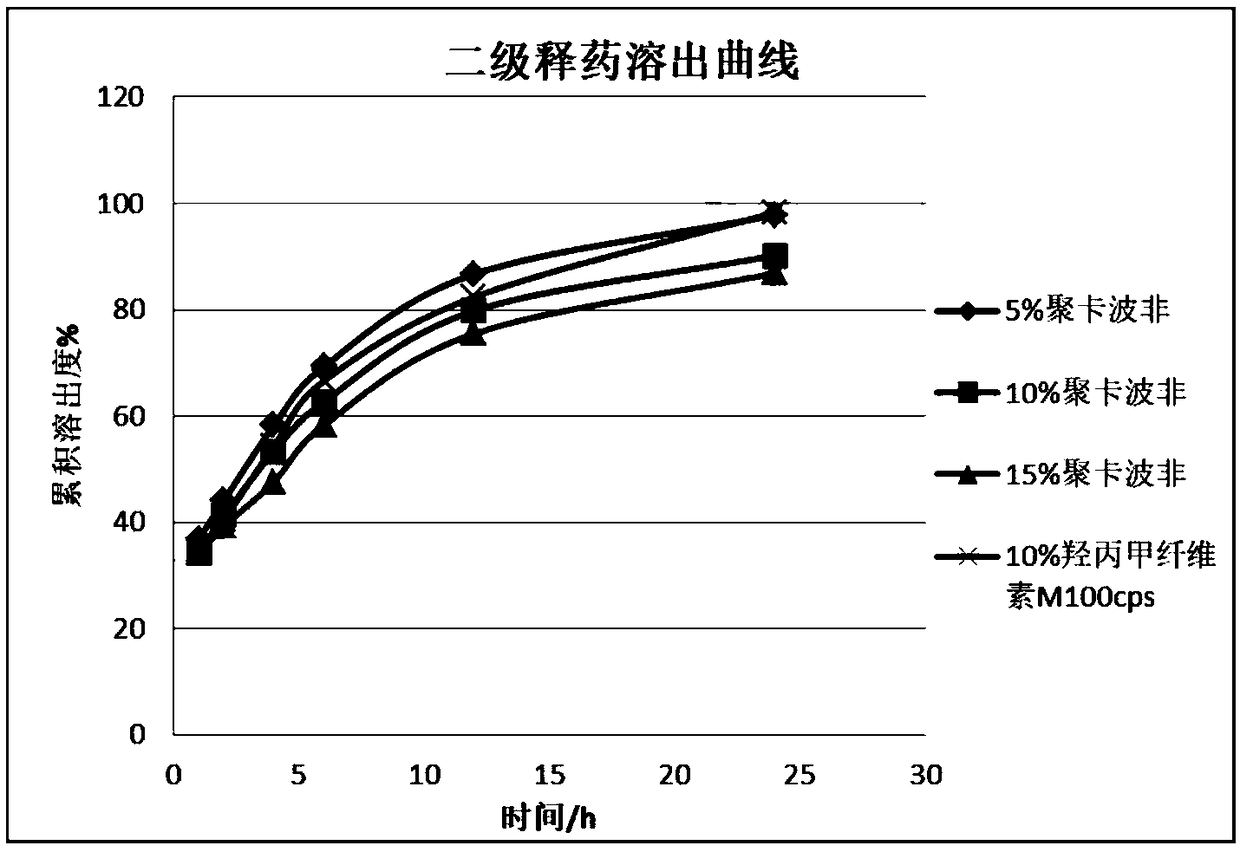
[0057] On the basis of the prescription and process preparation in the implementation case 1, the prescriptions were screened for different types and dosages, and followed up to investigate whether they had an impact on the adhesion performance, water swelling coefficient and drug dissolution. In embodiment 2, high viscosity hypromellose (M100cps) and polycarbophil were selected as adhesives, and the content of adhesives was selected as 5%, 10%, and 15%.
[0058] The prescription is as follows:
[0059] Each component and its mass ratio in the prescription 2-4 of table 3
[0060]
[0061] The preparation process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com