Kurthia gibsonii and application of Kurthia gibsonii in splitting of 1-phenyl-1,2-glycol
A technology of Kutia gigasii and ethylene glycol, which is applied in the directions of microorganism-based methods, bacteria and hydroxyl compounds separation/purification, etc., can solve the problems of low reaction substrate concentration, etc., and achieves low cost, mild reaction conditions, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Identification of the Molecular Biology Level of Kurthia gibsonii (Kurthia gibsonii) SC0312
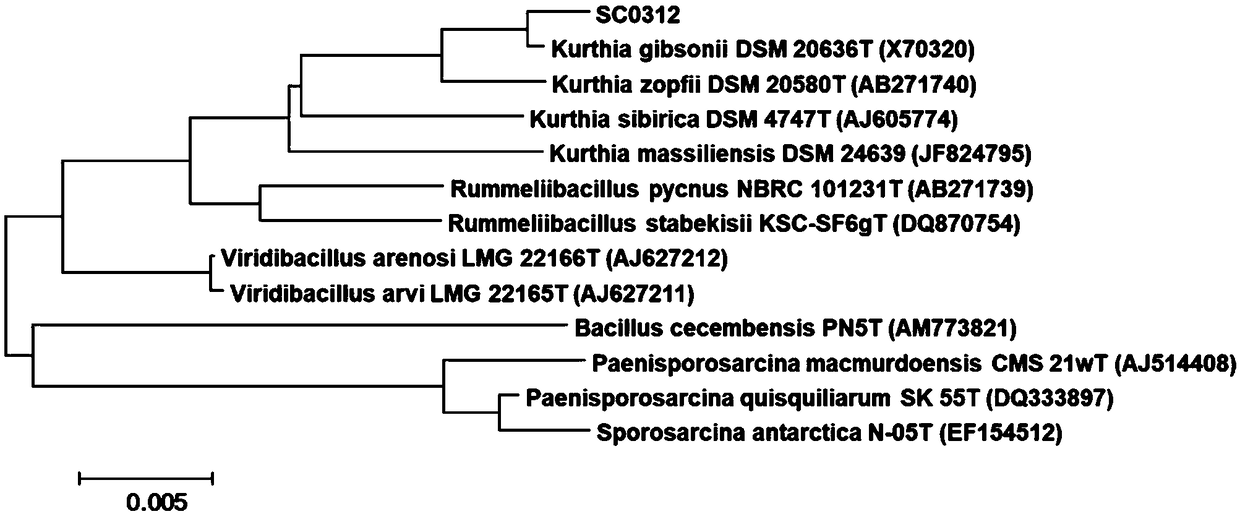
[0039] The Kurthia gibsonii SC0312 of the present invention is screened from local soil soaked in oil for a long time in Guangzhou. Among them, the strain phylogenetic tree such as figure 1 As shown, its 16S rDNA gene sequence determination results are as follows:
[0040] GAAATGCGGCAGCTATAATGCAAGTCGAGCGAATGACGAGAAGCTTGCTTCTCTGATTTAGCGGCGGACGGGTGAGTAACACGTGGGCAACCTGCCCTACAGATCGGGATAACTCAGGGAAACCTGGGCTAATACCGGATAATCCTTCGAATCACATGTTTTGAAGTTGAAAGGCGCTTCGGCGTCACTGTAGGATGGGCCCGCGGTGCATTAGCTAGTTGGTGGGGTAACGGCCTACCAAGGCAACGATGCATAGCCGACCTGAGAGGGTGATCGGCCACATTGGGACTGAGACACGGCCCAAACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCACAATGGACGAAAGTCTGATGGAGCAACGCCGCGTGAGTGATGAAGGTTTTCGGATCGTAAAACTCTGTTGTAAGGGAAGAACAAGTACGTTAGGAAATGAACGTACCTTGACGGTACCTTATTAGAAAGCCACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTGGCAAGCGTTGTCCGGATTTATTGGGCGTAAAGCGCGCGCAGGTGGTTTCTTAAGTCTGATGTGAAAGCCCACGGCTCAACCGTGGAGGGTCATTGGAA...
Embodiment 2
[0042] Embodiment 2 K.gibsonii (K.gibsonii) SC0312 culture process curve and catalytic activity change
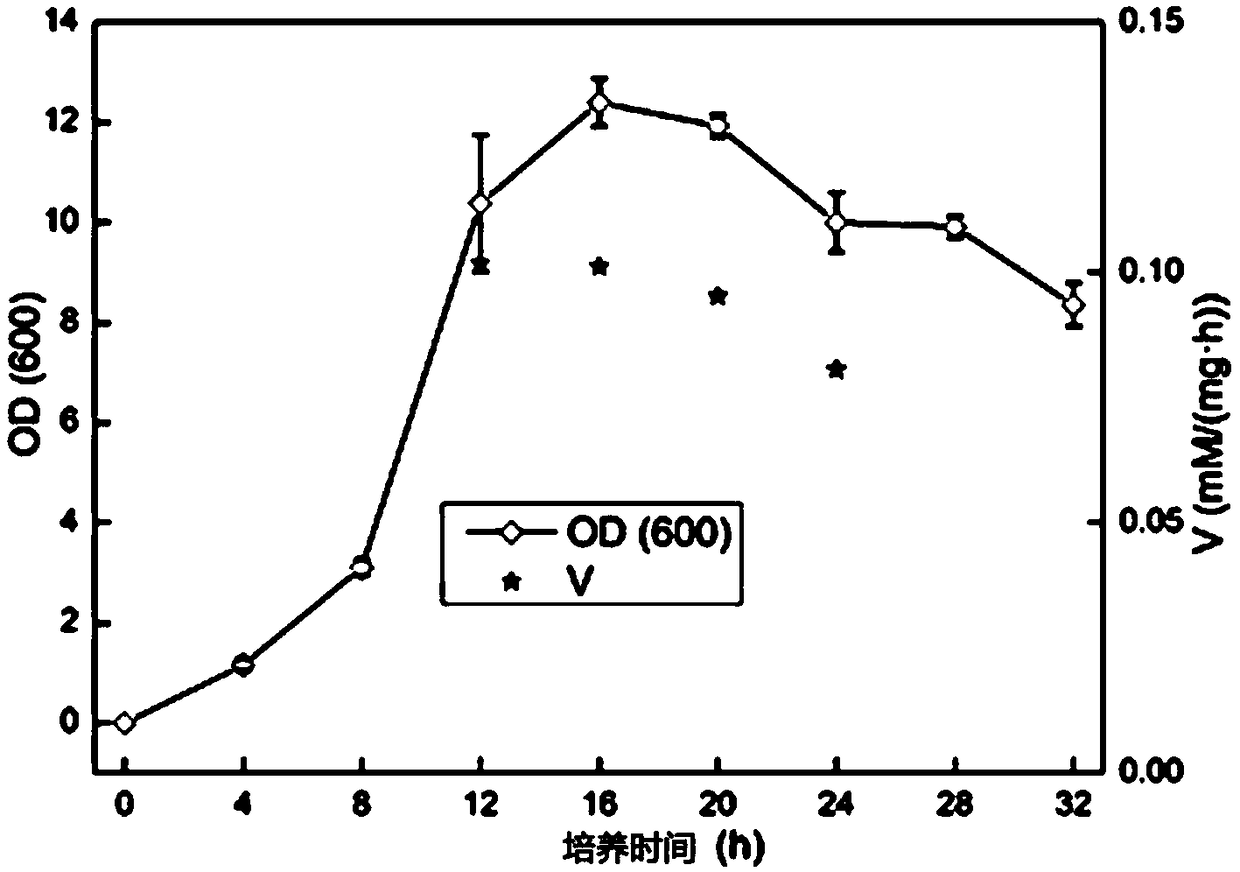
[0043] (1) Inoculate 0.1% (v / v) of the bacteria stored in the glycerol tube into 50ml of seed medium, culture at 180rpm and 37°C for 12h, then inoculate 0.1% (v / v) into 100ml In the fermentation medium of 180rpm, 37 ℃ for 0 ~ 32h. Monitor the change of OD (600nm) during the growth of the strain (measure every 4 hours). The result is as figure 2 shown.
[0044] (2) Centrifuge the bacteria that have been cultured in the fermentation medium for 12h, 16h, 20h, and 24h in step (1) at 4°C, collect the bacteria to obtain resting cells, and then recycle the harvested resting cells respectively. Suspend in phosphate buffer (100mM, pH 6.5), add racemic 1-phenyl-1,2-ethanediol (substrate 1-phenyl-1,2-ethanediol final concentration is 20mM ), wherein the final concentration of wet bacteria is 30mg / mL, the catalytic conditions are that the temperature is 35°C, and the rotation spee...
Embodiment 3
[0046] Example 3 Preparation of (S)-PED by K. gibsonii (K.gibsonii) SC0312
[0047] (1) Inoculate 0.1% (v / v) of the bacteria stored in the glycerol tube into 50ml of seed culture medium, culture at 180rpm and 37°C for 12h, then inoculate 0.1% (v / v) into 100mL culture medium at 180 rpm and 37°C for 16 hours.
[0048] (2) Collect the bacteria by centrifugation at 4°C, resuspend in phosphate buffer, add 1-phenyl-1,2-ethanediol to carry out catalytic reaction, and the reaction medium of the catalytic system is phosphate buffer (100mM , pH 5), the final concentration of substrate 1-phenyl-1,2-ethanediol is 20mM, the final concentration of wet bacteria is 30mg / mL, and the final concentration of acetone is 30mM. The catalytic condition is that the temperature is 45° C., and the rotation speed of the shaker incubator is 180 rpm. Samples were taken after catalysis for 11 hours, and the yield and enantiomeric excess of (S)-1,2-phenylethylene glycol (PED) were analyzed.
[0049] (3) A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com