Preparation and analysis methods of benzimidazole derivative
A technology of benzimidazole and its derivatives, which is applied in the field of preparation of pharmaceutical compounds, and can solve problems such as environmental pollution and potential safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
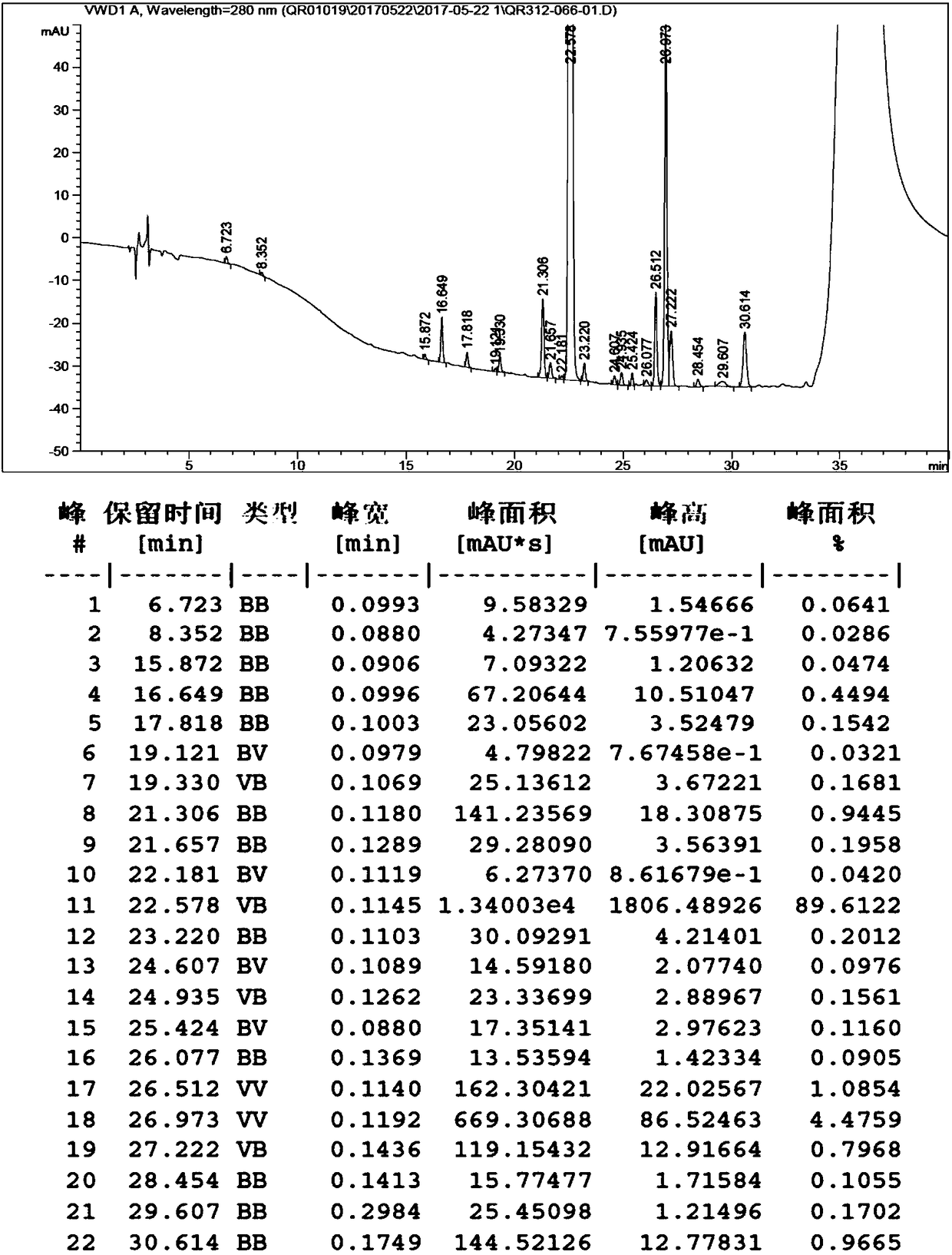
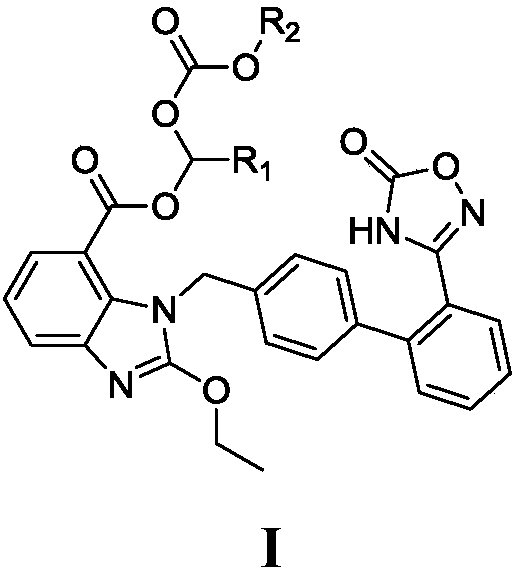
[0116] According to the method before the column chromatography described in Example 13 of patent application 201410010180.1, 10 kg of the pure compound of the following formula 4-1 was dissolved in 200 L of DMF, and 21.2 kg of azilsartan and 25 kg of cesium carbonate were added under stirring, and then the temperature was raised to 40°C, keep this temperature for 24 hours. TLC monitors the reaction and shows that the compound of formula 4-1 has almost reacted completely. The temperature of the reaction solution is lowered to room temperature, and 1000L of water is added, extracted with ethyl acetate (200L*3), and the combined organic phases are washed with saturated aqueous sodium chloride (100L*2 ), dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain a 20kg mixture, which is an oil containing a compound of formula 5 and its rearrangement impurity compound 5-1, wherein the content of the rearrangement impurity compound 5-1 is detecte...
Embodiment 1
[0119] 1) Add 40 L of butanol to about 10 kg of the crude oil of the compound of formula 5, heat up to 80° C. to dissolve, cool to room temperature and crystallize overnight. Filtrate, collect the solid, rinse the filter cake with 2.0L butanol, and dry under reduced pressure to obtain 9.5 kg of the product, with a yield of 95.0% (calculated based on the crude oil of the compound of formula 5), and a product purity of 87.0%;
[0120] 2) Add 18.4L of tert-butylketone to 9.5kg of solid compound of formula 5 after recrystallization in the previous step, beat at room temperature for 2h, filter, and dry under reduced pressure to obtain 8.64kg of product with a purity of 99.2% and a yield of 90.9%, in which the content of rearranged impurities 0.42%, less than 0.50%.
Embodiment 2
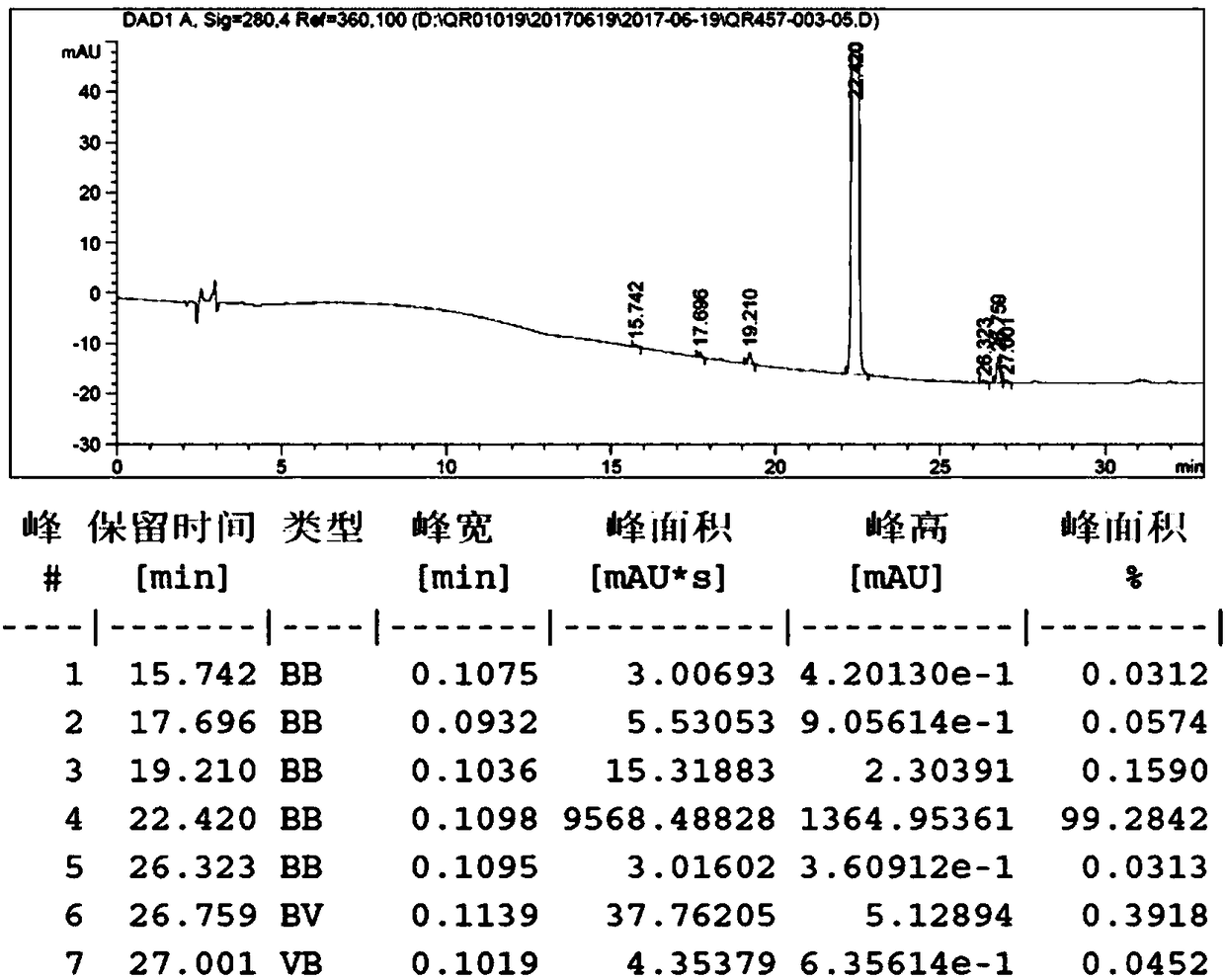
[0122] 1) Add 52L of ethanol with a mass percentage of 75% to about 13kg of the crude oil of the compound of formula 5, and heat up to 60°C for 2 hours to dissolve. Slowly cool down to room temperature to crystallize overnight. Filter to collect the solid, rinse the filter cake with 2L of 75% ethanol, and dry under reduced pressure to obtain 11.1 kg of the product, with a yield of 85.4% (calculated based on the crude oil of the compound of formula 5), and a product purity of 87.6%.
[0123] 2) Add 110L of methyl isobutyl ketone to the solid 11.1Kg of the compound of formula 5 after recrystallization in the previous step, heat to 80°C to completely dissolve, cool to room temperature for crystallization overnight, filter, and dry under reduced pressure to obtain 9.6 kg of the compound of formula 5 , the purity is 99.4%, the yield is 86.5%, and the rearrangement impurity content is 0.38%, less than 0.50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com