Organic light emitting device with pyridine compounds
An organic light-emitting device, azabenzene technology, applied in the direction of light-emitting materials, organic chemistry, electric solid-state devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
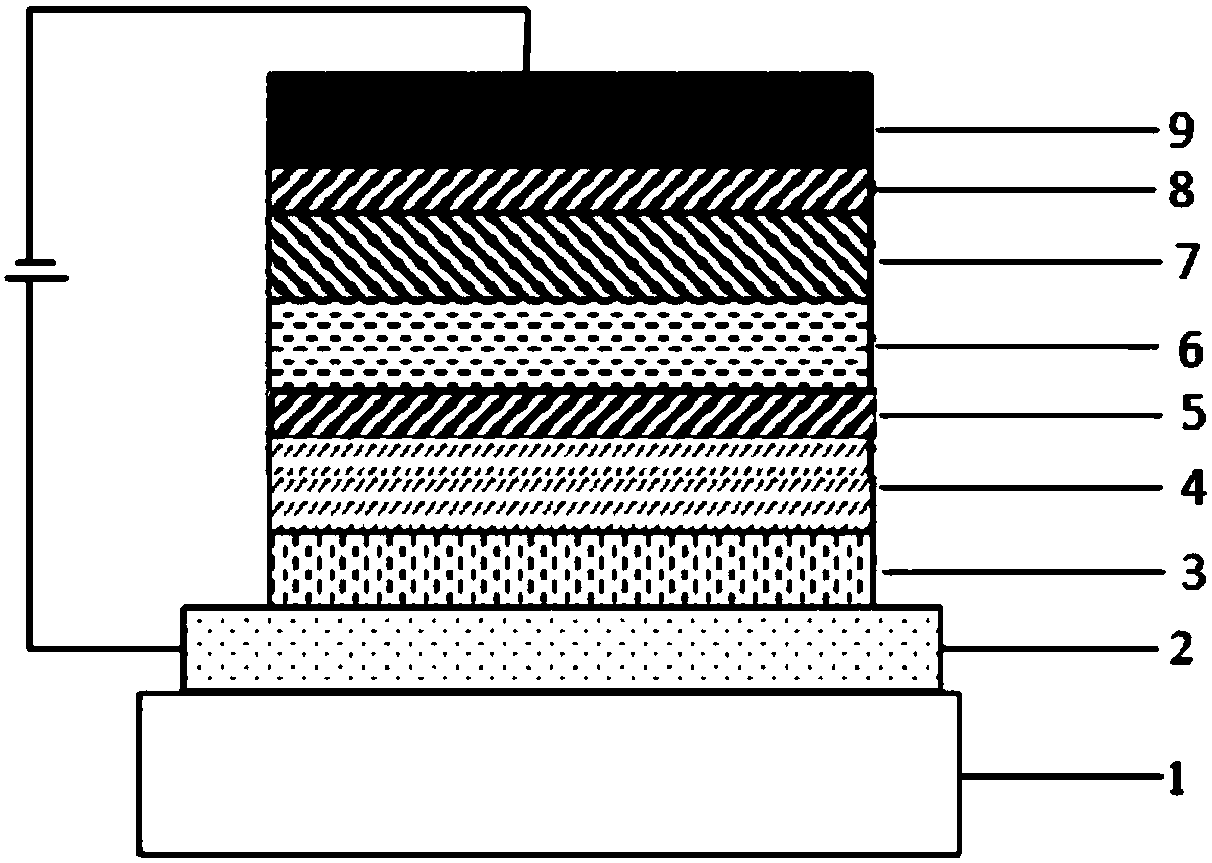
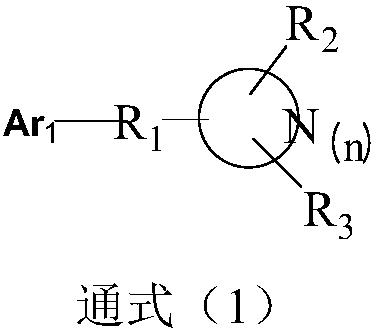
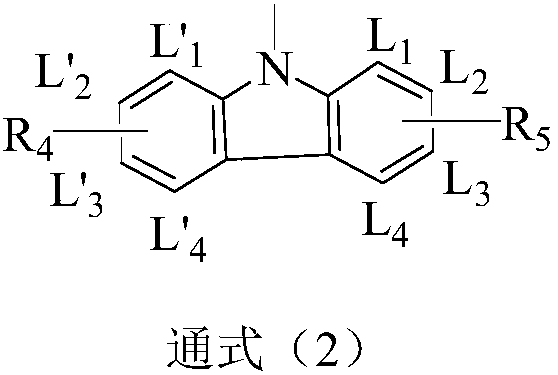
Image
Examples
preparation example Construction
[0079] Concrete preparation method is:
[0080] 1) Dissolving raw material I and intermediate A with toluene, the amount of toluene is 30-50mL toluene per gram of raw material I, wherein the molar ratio of raw material I to intermediate A is 1:(1.2~4.5);
[0081] 2) Add Pd to the reaction system in 1) 2 (dba) 3 , triphenylphosphine and potassium tert-butoxide; the Pd 2 (dba) 3 The mol ratio with raw material I is (0.006~0.02): 1, the mol ratio of described triphenylphosphine and raw material I is (0.006~0.02): 1, and the mol ratio of described sodium tert-butoxide and raw material I is ( 2.0~5.0): 1;
[0082] 3) Under the protection of an inert gas, react the above mixed solution at 95-110°C for 10-24 hours, cool naturally to room temperature, and filter the reaction solution. The filtrate is rotary-evaporated under reduced pressure until there is no solvent, and passed through a neutral silica gel column to obtain the target product, the condition of the vacuum rotary ev...
Embodiment 1
[0087] Embodiment 1: the synthesis of compound 1
[0088]
[0089] Weigh 0.01mol of raw material 1 and 0.015mol of intermediate A and dissolve in 150mL of anhydrous toluene, add 0.02mol of sodium tert-butoxide, 0.02mol of triphenylphosphine and 10 -4 mol Pd 2 (dba) 3 , heated to reflux for 12 hours, sampling point plate, the raw material reacted completely; naturally cooled to room temperature (20 ~ 25 ° C), filtered, collected the filtrate and subjected to reduced pressure rotary evaporation (-0.09MPa, 85 ° C), and performed column chromatography to obtain the target The product has an HPLC purity of 99.5% and a yield of 72.4%.
[0090] Elemental analysis structure (molecular formula C 41 h 26 N 2 O): theoretical value C, 87.52; H, 4.66; N, 4.98; found value: C, 87.43; H, 4.71; N, 4.94. ESI-MS(m / z)(M + ): The theoretical value is 562.66, and the measured value is 562.87.
Embodiment 2
[0091] Embodiment 2: the synthesis of compound 23
[0092]
[0093] The synthetic procedure of compound 23 is similar to that of compound 1, except that intermediate A is replaced by intermediate B.
[0094] Elemental analysis structure (molecular formula C 44 h 32 N 2 ): theoretical value C, 89.76; H, 5.48; N, 4.76; test value: C, 89.65; H, 5.44; N, 4.81. ESI-MS(m / z)(M + ): The theoretical value is 588.74, and the measured value is 588.25.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com