Hydrogenation dehalogenation method of halogenated aromatic hydrocarbon
A halogenated aromatic hydrocarbon and hydrodehalogenation technology, applied in the field of hydrodehalogenation of halogenated aromatic hydrocarbons, can solve the problems of high bond energy, difficult catalysis, low applicability, etc., achieve low toxicity, easy degradation, and reduce metal salts the effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
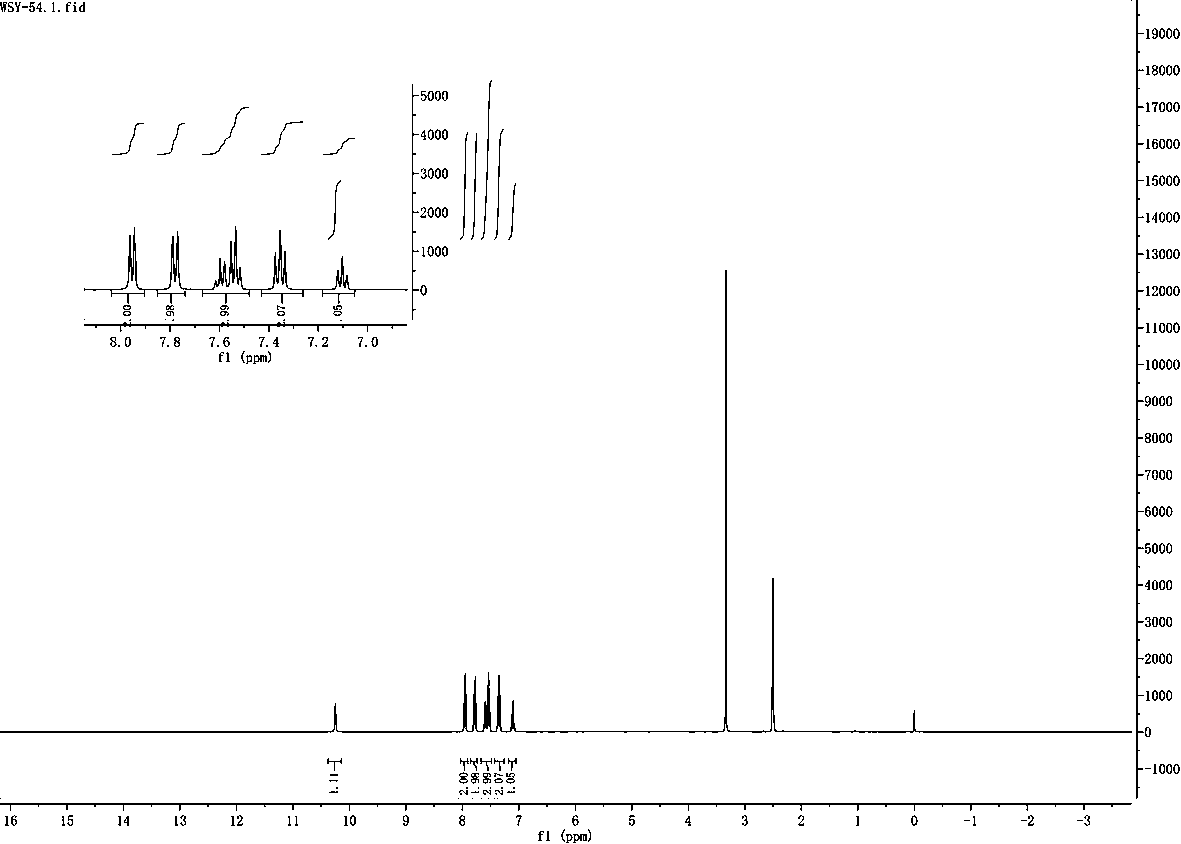
[0031] The hydrodechlorination of embodiment 1 o-chlorobenzoic acid
[0032] Step 1: Weigh 78mg (0.5mmol%) of o-chlorobenzoic acid, 34mg (0.5mmol) of imidazole, 24mg (12mmol%) of cupric ammonium sulfate (hexahydrate), 11mg (2mmol%) of rose bengal, lithium tert-butoxide t- BuOli 72mg (0.9mmol) in a quartz reaction tube. Add 1.5 ml each of isopropanol and DMF as solvent.
[0033] Step 2: Place the quartz reaction tube under 254nm ultraviolet light for 72 hours under air condition. The reaction formula is as follows:
[0034]
[0035] Step 3: After the reaction was completed, the product was separated by column chromatography, and the yield was 87%. The resulting product is benzoic acid.
Embodiment 2
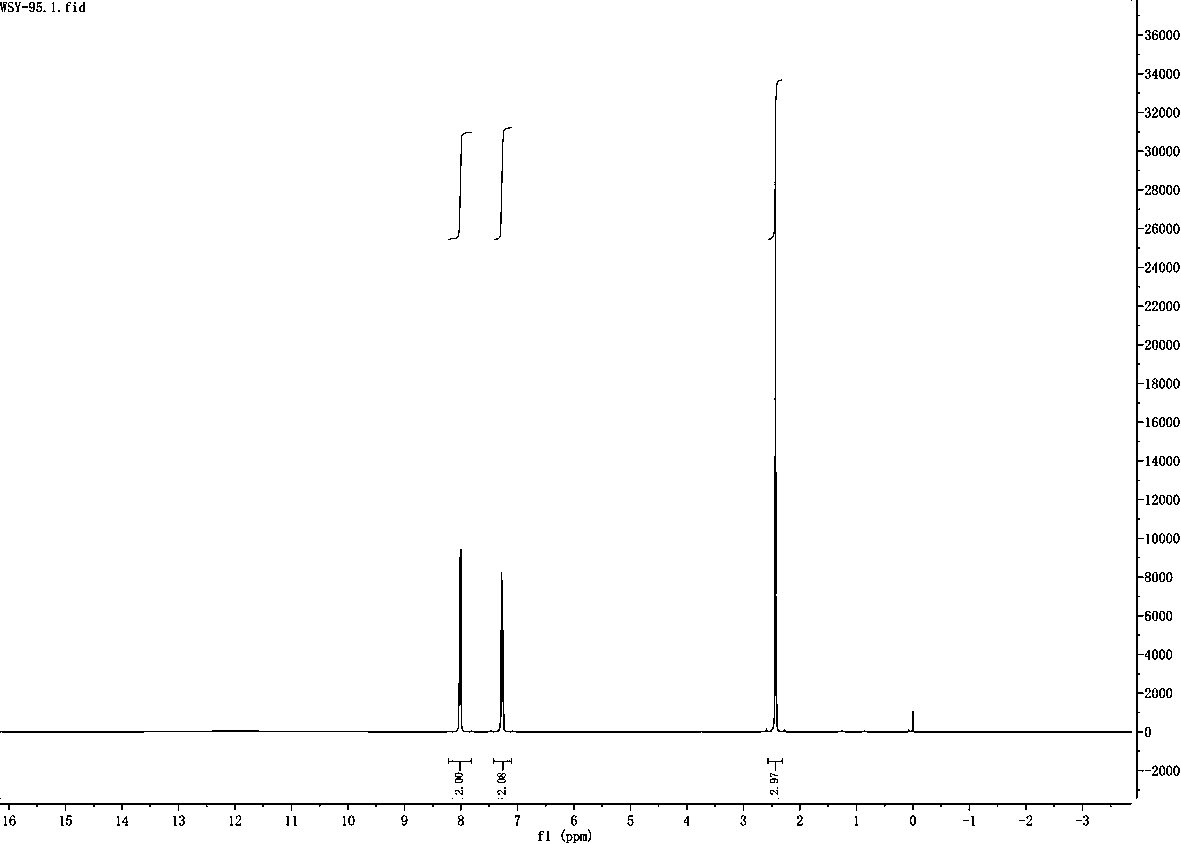
[0036] Example 2 Hydrodechlorination of 2-chloro-nitrogen-phenylbenzamide
[0037] Step 1: Weigh 116mg (0.5mmol) of 2-chloro-nitrogen-phenylbenzamide, 34mg (0.5mmol) of imidazole, 24mg (12mmol%) of copper ammonium sulfate (hexahydrate), and 11mg (2mmol%) of rose bengal , Lithium tert-butoxide t-BuOli 72mg (0.9mmol) in a quartz reaction tube. Add 1.5 ml each of isopropanol and DMF as solvent.
[0038] Step 2: Place the quartz reaction tube under 254nm ultraviolet light for 72 hours under air condition. The reaction formula is as follows:
[0039]
[0040] Step 3: After the reaction was completed, the product was separated by column chromatography, and the yield was 87%. The resulting product is nitrogen-phenylbenzamide.
Embodiment 3
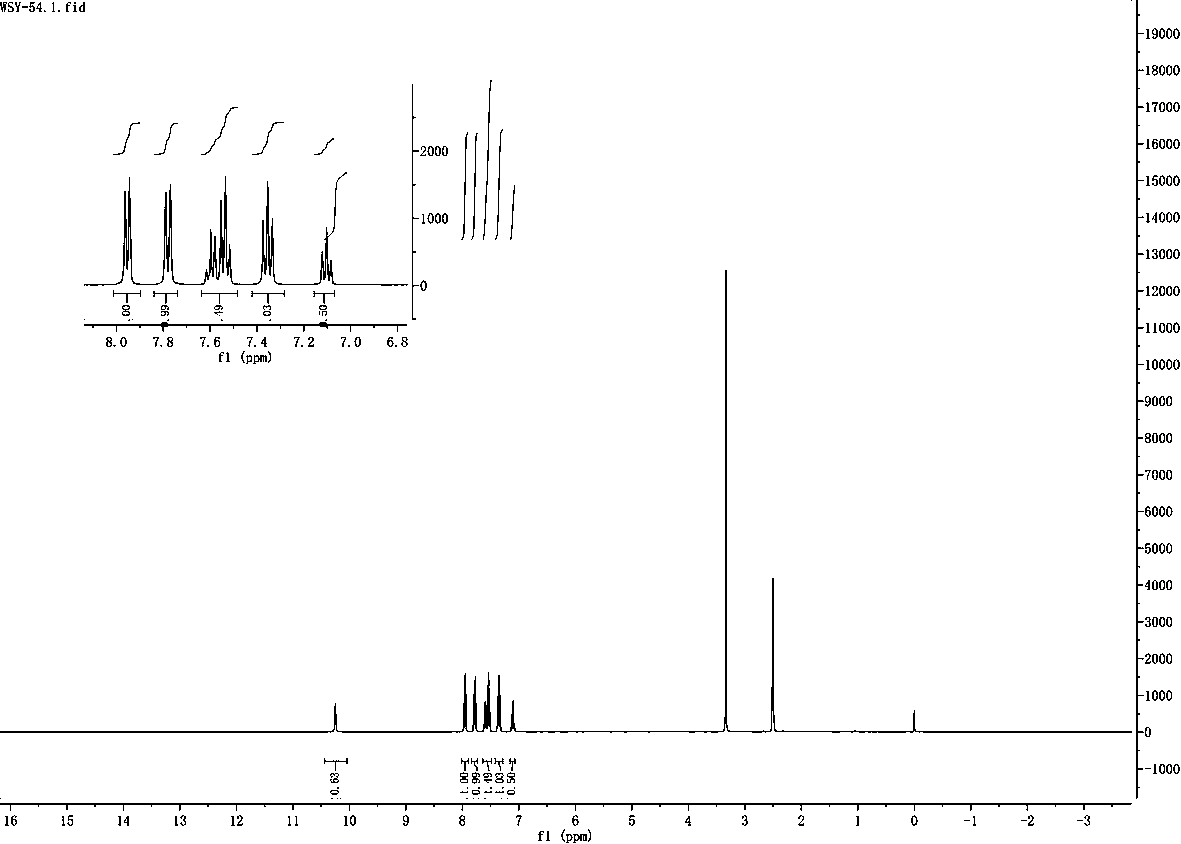
[0041] Example 3 The hydrodechlorination of 2-chloro-4-methylbenzoic acid
[0042] Step 1: Weigh 85mg (0.5mmol) of 2-chloro-4-methylbenzoic acid, 34mg (0.5mmol) of imidazole, 24mg (12mmol%) of cupric ammonium sulfate (hexahydrate), 11mg (2mmol%) of rose bengal, Lithium tert-butoxide t-BuOli 72mg (0.9mmol) in a quartz reaction tube. Add 1.5 ml each of isopropanol and DMF as solvent.
[0043] Step 2: Place the quartz reaction tube under 254nm ultraviolet light for 72 hours under air condition. The reaction formula is as follows:
[0044]
[0045] Step 3: After the reaction was completed, the product was separated by column chromatography, and the yield was 85%. The resulting product is p-toluic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com