Multifunctional polyurethane derivative containing triarylamine structure and tetrastyrene group as well as preparation method and application thereof.
A technology of polyurethane derivatives and tetraphenylethylene, applied in the preparation of urea derivatives, preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of few functional types, and achieve accelerated processing and application, high temperature resistance, and high heat The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
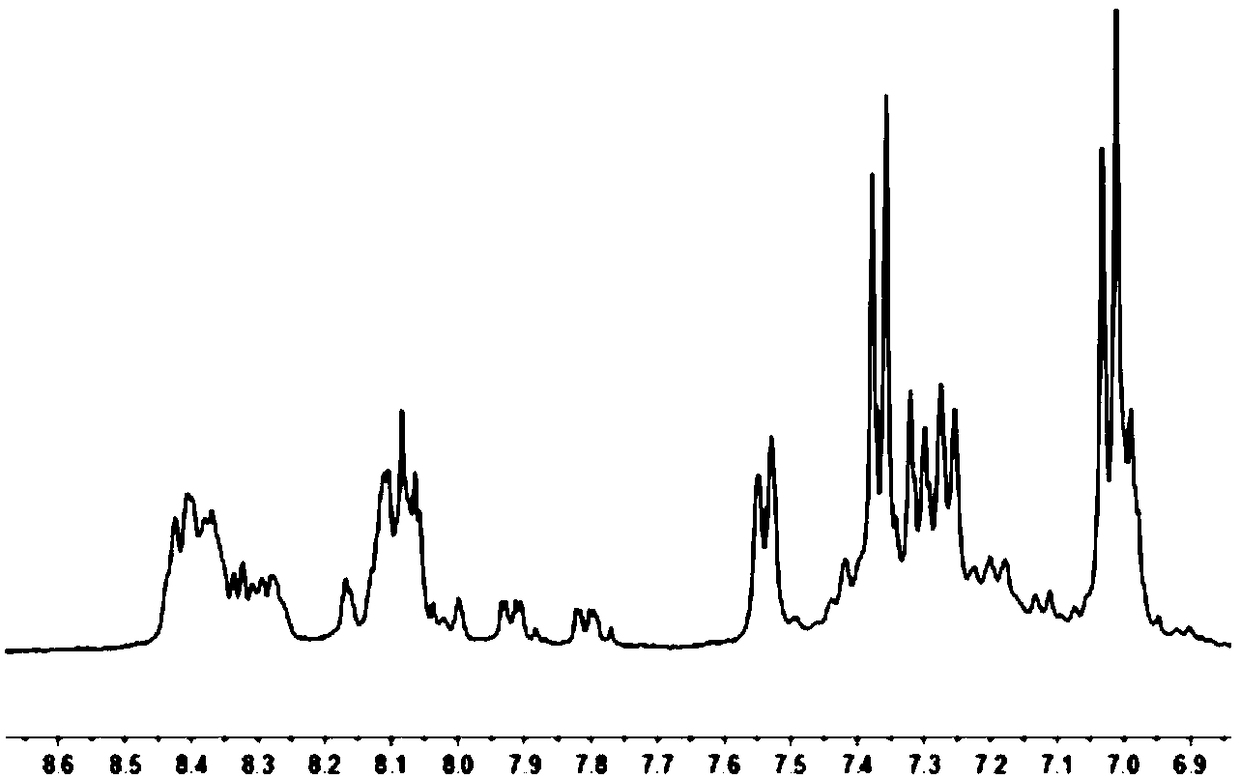
[0044] Specific embodiment 1: In this embodiment, the multifunctional polyurethane derivative containing triarylamine structure and tetraphenylethylene group is N, N'-bis(4-aminophenyl)-N, N'-diphenyl-1 , Multifunctional polyurethane derivatives of 4-phenylenediamine and tetraphenylethylene groups or N, N'-bis(4-aminophenyl)-N, N'-diphenyl-1,4-biphenyl Multifunctional polyurethane derivatives of amine groups and tetraphenylethylene groups;
[0045] The structural formula of the multifunctional polyurethane derivative containing N, N'-bis(4-aminophenyl)-N, N'-diphenyl-1,4-phenylenediamine and tetraphenylethylene group is as follows:
[0046] In the formula, n is an integer of 3 to 10;
[0047] The structural formula of the multifunctional polyurethane derivative containing N, N'-bis(4-aminophenyl)-N, N'-diphenyl-1,4-biphenylenediamine group and tetraphenylethylene group is as follows:
[0048] ,
[0049] In the formula, n is an integer of 3-10.
[0050] In this embodime...
specific Embodiment approach 2
[0053] Specific implementation mode two: the method of the multifunctional polyurethane derivative containing triarylamine structure and tetraphenylethylene group in this implementation mode is as follows: 1. 1,2-bis(4-hydroxyphenyl)-1,2-diphenyl Ethylene, 4,4'-diphenylmethane diisocyanate and solvent N,N-dimethylacetamide (DMAC) are mixed, stirred and refluxed at room temperature for 5-15 hours, and the stirring speed is 800r / min~900r / min, to obtain Precursor; 2. It will contain N, N'-bis(4-aminophenyl)-N,N'-diphenyl-1,4-phenylenediamine or N,N'-bis(4-aminophenyl) )-N,N'-diphenyl-1,4-biphenyldiamine diamine monomer is added to the precursor prepared in step 1, the temperature is raised to 70-120°C, stirred and refluxed for 5-15h, cooled and poured into in methanol, and then filtered, and the obtained solid phase is vacuum-dried to obtain a multifunctional polyurethane derivative containing a triarylamine structure and a tetraphenylethylene group;
[0054] Which contains N,N'...
specific Embodiment approach 3
[0056] Specific embodiment 3: The difference between this embodiment and specific embodiment 2 is that it contains N,N'-bis(4-aminophenyl)-N,N'-diphenyl-1,4-phenylenediamine or N , N'-bis(4-aminophenyl)-N,N'-diphenyl-1,4-biphenylenediamine diamine monomer, 1,2-bis(4-hydroxyphenyl)-1 , 2-diphenylethylene, 4,4'-diphenylmethane diisocyanate molar ratio: 2:1:2. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com