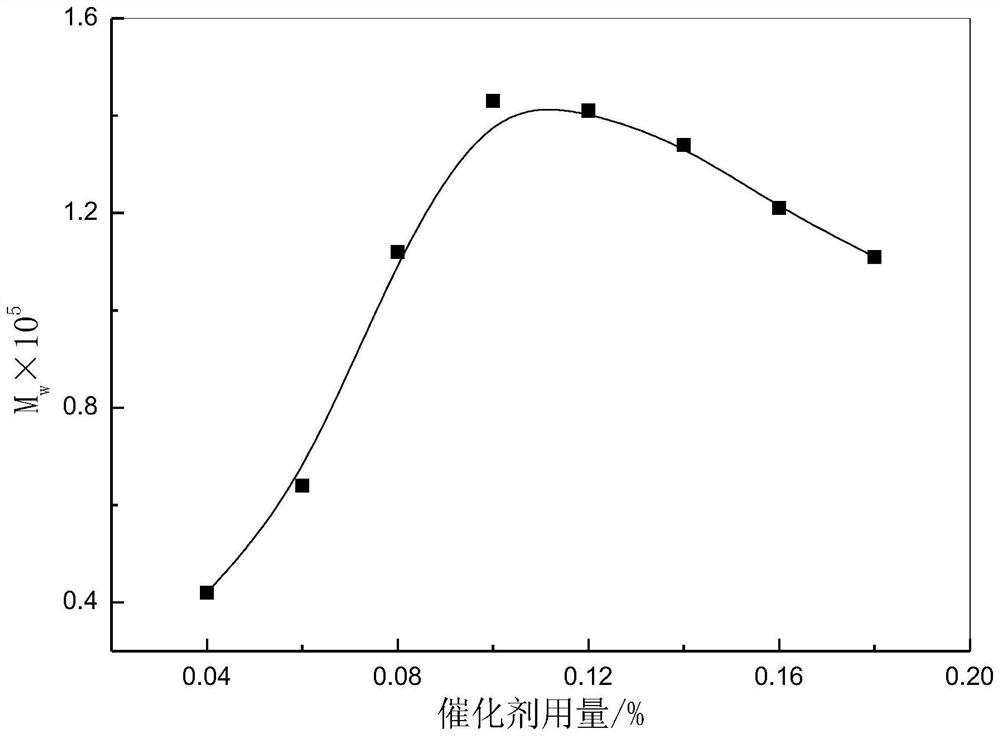
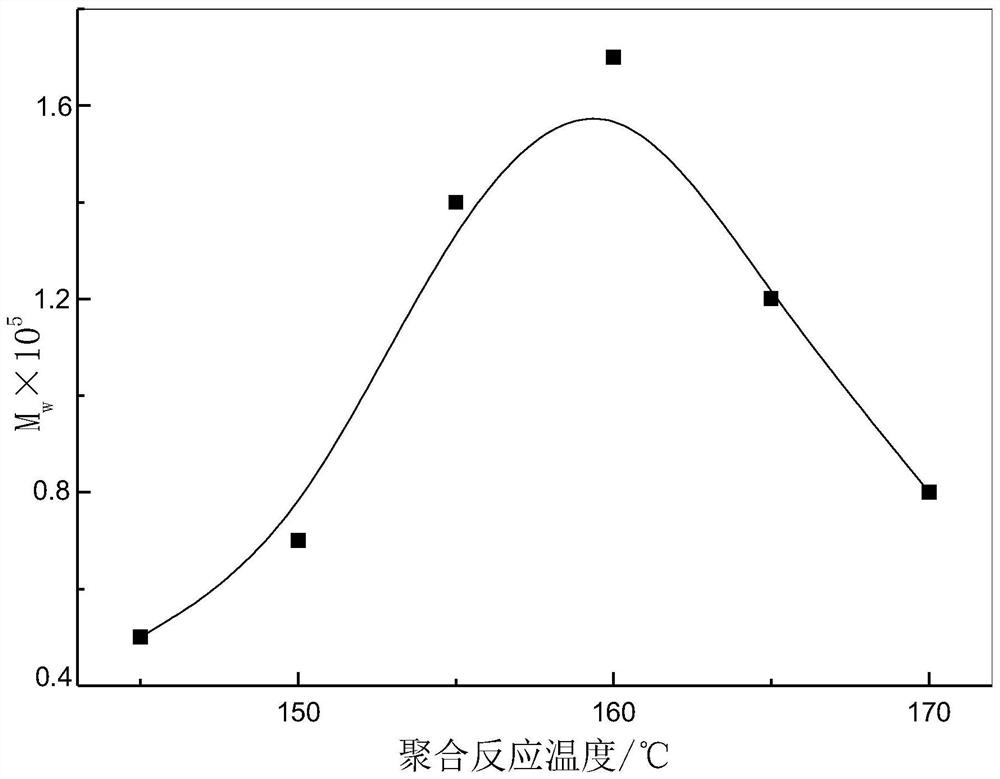
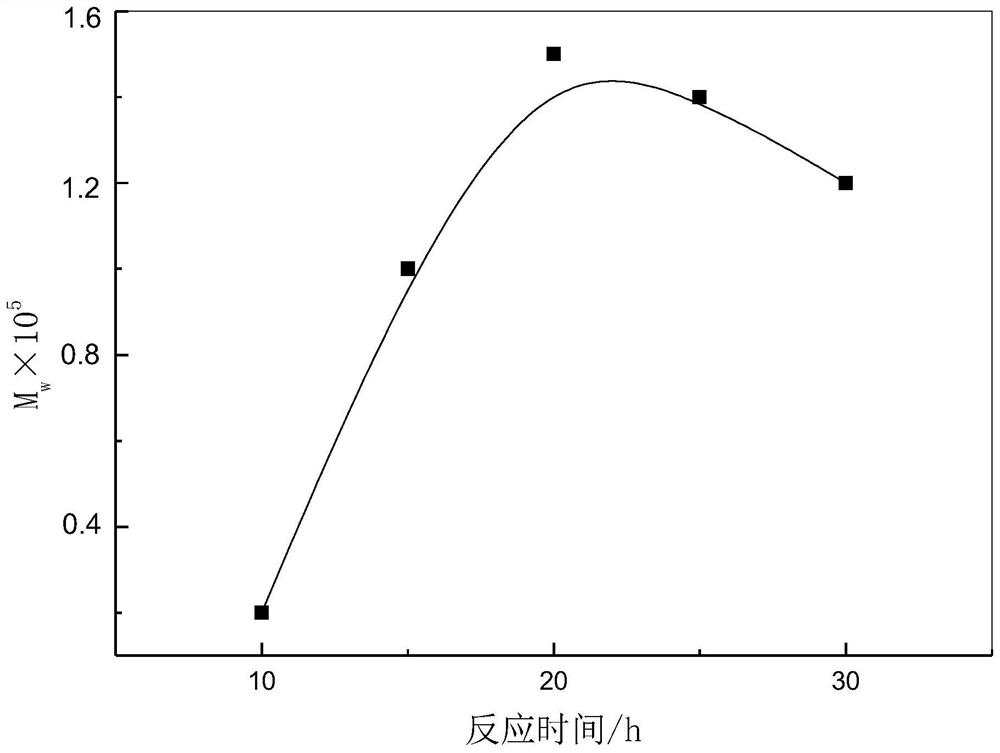
Method for producing poly-l-lactic acid by ring-opening polymerization of l-lactide
A technology of ring-opening polymerization and lactide, which is applied in the field of poly-L-lactic acid production, can solve problems such as unsuitable for industrial application, high cost, and toxic solvents, and achieve high reaction pressure, energy saving, high yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] A method utilizing L-lactic acid to produce L-lactide, comprising the steps of:
[0064] (1) Raw material preparation: 45wt% L-lactic acid solution produced by the applicant, the optical purity of L-lactic acid is greater than or equal to 99.5%;
[0065] (2) Dehydration oligomerization to obtain L-lactic acid oligomers;
[0066] Add ethyl pyruvate to the L-lactic acid solution and stir to obtain a mixed solution, the volume ratio of ethyl pyruvate to the L-lactic acid solution is 1:1;
[0067] Simultaneously add cobalt oxide and two-cysteine methyl ester vanadyl as catalyst in mixed solution according to the following steps:
[0068] During the initial reaction (0h): the mass ratio of cobalt oxide and di-cysteine methyl ester vanadyl is 1:1.5, and the amount of cobalt oxide added during the initial reaction is 1 / 2 of the total amount of cobalt oxide added; The reaction temperature is kept at 5°C from the initial reaction to half of the reaction time; stirring;
...
Embodiment 2
[0083] A method utilizing L-lactic acid to produce L-lactide, comprising the steps of:
[0084] (1) Raw material preparation: 60wt% L-lactic acid solution produced by the applicant, the optical purity of L-lactic acid is greater than or equal to 99.5%;
[0085] (2) Dehydration oligomerization to obtain L-lactic acid oligomers;
[0086] Add ethyl pyruvate to L-lactic acid solution and stir to obtain a mixed solution, the volume ratio of ethyl pyruvate to L-lactic acid solution is 1:1; add cobalt oxide and di-cysteine methyl ester to the mixed solution Vanadyl is used as a catalyst; the amount of cobalt oxide used is 0.04% of the mass of L-lactic acid, the amount of di-cysteine methyl ester vanadyl is 0.08% of the mass of L-lactic acid, and the reaction is stirred for 3 hours at room temperature; The preparation method of vanadyl methyl ester is as follows: methyl cysteine and vanadyl sulfate are mixed and stirred at room temperature in boric acid solution, and react for ...
Embodiment 3
[0098] A method utilizing L-lactic acid to produce L-lactide, comprising the steps of:
[0099] (1) Raw material preparation: 50wt% L-lactic acid solution produced by the applicant, the optical purity of L-lactic acid is greater than or equal to 99.5%;
[0100] (2) Dehydration oligomerization to obtain L-lactic acid oligomers;
[0101] Add ethyl pyruvate to the L-lactic acid solution and stir to obtain a mixed solution, the volume ratio of ethyl pyruvate to the L-lactic acid solution is 1:1;
[0102] Simultaneously add cobalt oxide and two-cysteine methyl ester vanadyl as catalyst in mixed solution according to the following steps:
[0103] During the initial reaction (0h): the mass ratio of cobalt oxide and di-cysteine methyl ester vanadyl is 1:1.5, and the amount of cobalt oxide added during the initial reaction is 1 / 2 of the total amount of cobalt oxide added; The reaction temperature is kept at 10°C from the initial reaction to half of the reaction time; stirring;
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com