Non-isocyanate polyurethane preparation method
A non-isocyanate and polyurethane technology, applied in the field of bio-based material synthesis, can solve the problems of poor modifiability, consumption of fossil raw materials, and few kinds of raw materials, and achieve the effect of many kinds and strong modifiability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
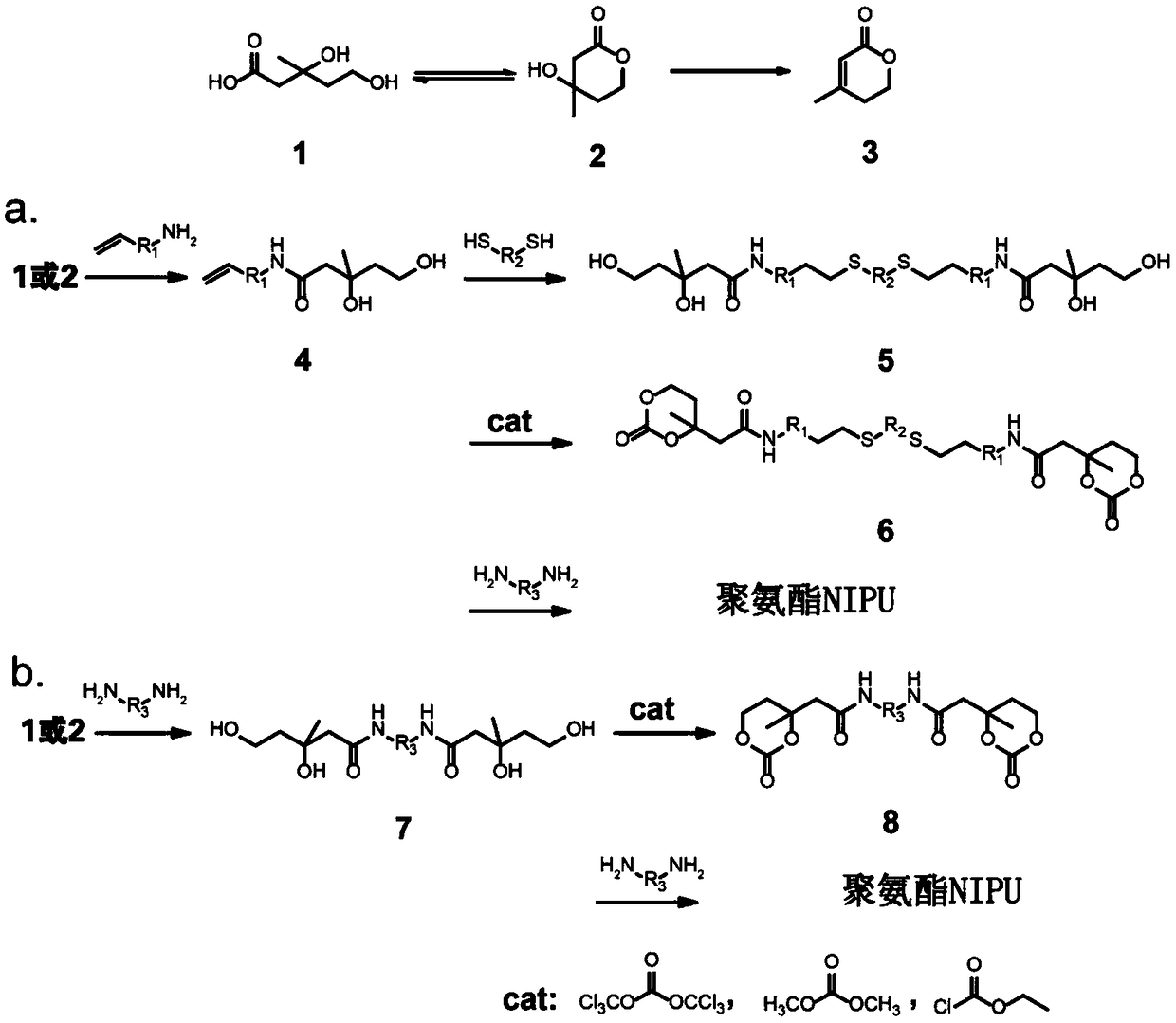
[0041] Embodiment 1. The method for preparing non-isocyanate polyurethane with mevalonolactone as substrate.
[0042] 1) Preparation of polyol derivatives: ethylenedithiol-bridged mevalonate propylamine dimer.
[0043] a. mevalonate to prepare allylamine mevalonate: mevalonate 1.0g (7.7mmol, 1eq), 3-aminopropene 4.4g (10eq) were dissolved in 30mL of dry m-xylene , stirred and reacted at 100° C. for 2 h. After the reaction was complete, the solvent was removed under reduced pressure to obtain a crude product. The crude product was separated by column chromatography to obtain 1.3 g of pure allylamine mevalonate with a yield of 90.3%. The reaction equation is:
[0044] b. Preparation of ethylenedithiol bridged mevalonate propylamine dimer: 1.0g (5.3mmol, 1eq) of mevalonate allylamine prepared in a, ethanedithiol 0.246g (0.5eq) It was dissolved in 30 mL of dry dichloromethane, placed in a dark environment, stirred and reacted at 25°C for 10 h under ultraviolet light irradiatio...
Embodiment 2
[0051] Embodiment 2. The method for preparing non-isocyanate polyurethane with mevalonolactone as substrate.
[0052] 1) Preparation of polyol derivative ethylenediamine-bridged mevalonate dimer: take 1.0g (7.7mmol, 1.0eq) of mevalonate, dissolve 0.23g (0.5eq) of ethylenediamine in 30mL and dry In m-xylene, stirred and reacted at 100°C for 2 hours, after the reaction was complete, the solvent was removed under reduced pressure to obtain a crude product, which was separated by column chromatography to obtain 2.1 g of pure ethylenediamine-bridged mevalonic acid dimer, producing Rate 85.0%. The reaction equation is as follows:
[0053]
[0054] 2) Preparation of ethylenediamine-bridged six-membered ring carbonate dimer
[0055] Dissolve 2.0 g (6.3 mmol, 1 eq) of the ethylenediamine-bridged mevalonic acid dimer prepared in step 1) in 20 mL of dry dichloromethane, and cool the mixture to 0° C. under nitrogen protection, then Add 5 g of excess dimethyl carbonate to the mixed s...
Embodiment 3
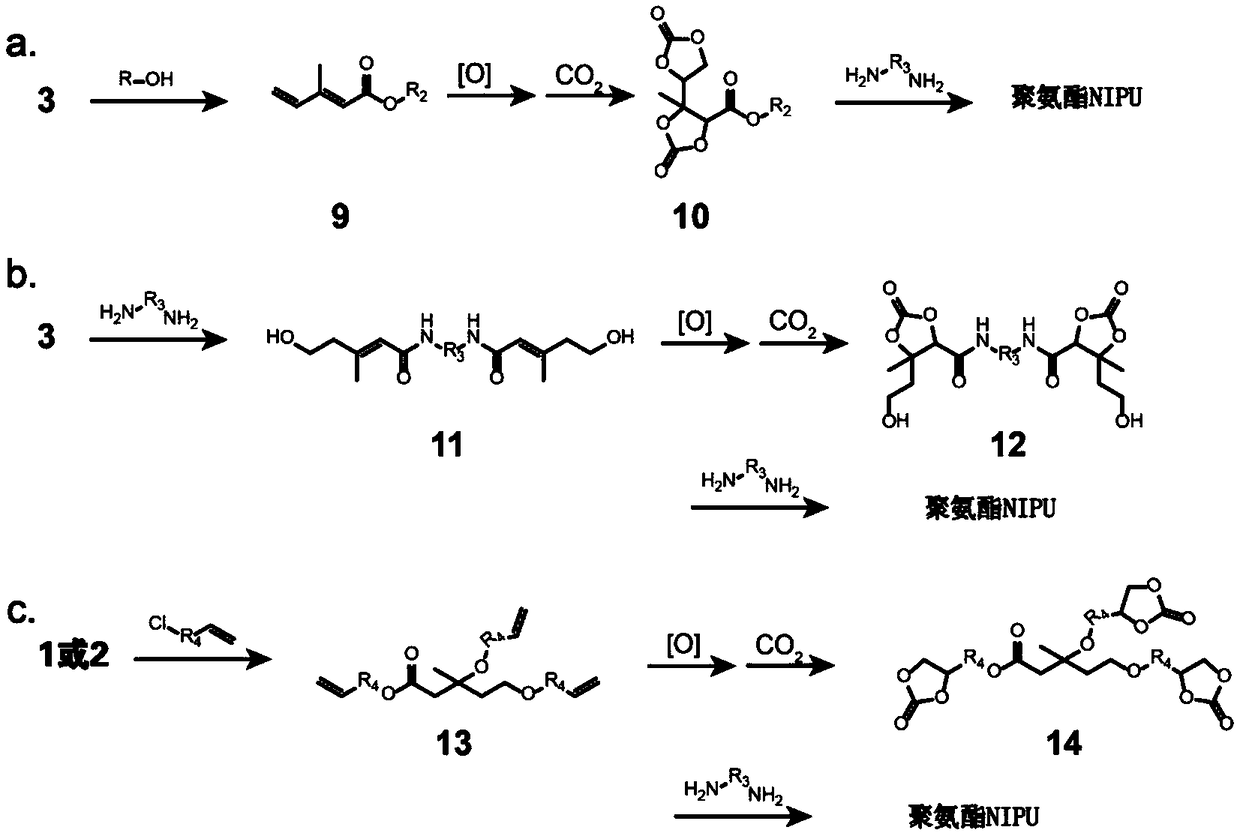
[0059] Embodiment 3. The method for preparing non-isocyanate polyurethane with mevalonolactone as substrate.
[0060] 1) Preparation of polyolefin derivative triallyl mevalonate:
[0061] Dissolve 1.0 g (7.7 mmol, 1 eq) of mevalonate and 1.8 g (3 eq) of 3-chloropropene in 30 mL of dry tetrahydrofuran, add 1.0 g of NaOH, and reflux at 80°C for 2 h. After the reaction was over, 50 mL of ice water was added to the reaction solution, followed by extraction with dichloromethane, rinsing with 100 mL of ultrapure water three times, Na 2 SO 4 After drying, the solvent was removed under reduced pressure to obtain 2.0 g of triallyl mevalonic acid with a yield of 96.9%. The reaction equation is as follows:
[0062]
[0063] 2) Preparation of tricyclic carbonate mevalonate polyurethane precursor.
[0064] Take 2.0 g (7.5 mmol, 1 eq) of triallyl mevalonic acid prepared in step 1) and dissolve it in 30 mL of tetrahydrofuran, add 5.0 g of silver-loaded silica nanospheres, react at 80°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com