Carbonyl reductase mutant mut-AcCR(l147V/G152L) and application and coding genes thereof
An I147V, mut-accr technology, applied in the field of enzyme molecule modification, can solve the problems of poor substrate tolerance and low enzyme activity, and achieve the goal of overcoming low enzyme activity, high enzyme activity and substrate tolerance, and overcoming substrate resistance. poorly tolerated effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The gene sequence of AcCR was translated into its amino acid sequence by standard methods, and the sequence was searched in the PDB database, and the three-level homology of 4RF2, 1ZJY, 1NXQ and 1ZK3 were selected with 53%, 51%, 51% and 51% homology respectively. Using the structure as a template, homology modeling and energy minimization were performed to obtain the tertiary structure model of carbonyl reductase AcCR. Further use Ramachandran Plot and Profile-3D to evaluate the structural rationality of each amino acid residue in the homology modeling results and the matching degree between the protein model and the amino acid sequence of the protein. It is determined that the model built is reasonable and can be used for subsequent experimental analysis. The tertiary structure of carbonyl reductase AcCR was docked with the coenzyme NADH, and the mutation hotspots of carbonyl reductase were predicted by HotSpot2.0, and the 147I and 152G sites were used as mutation site...
Embodiment 2
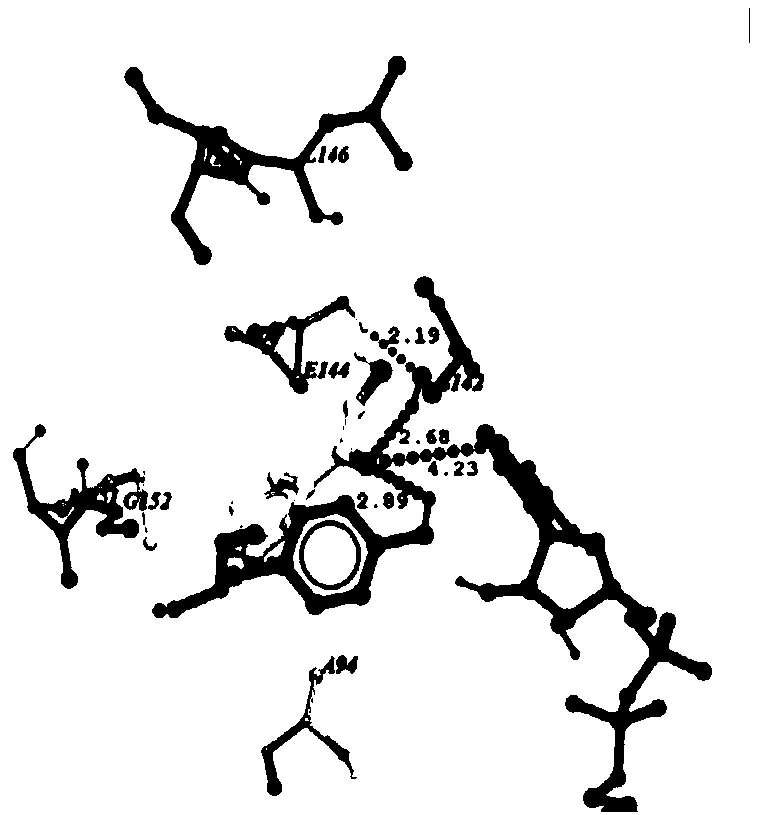
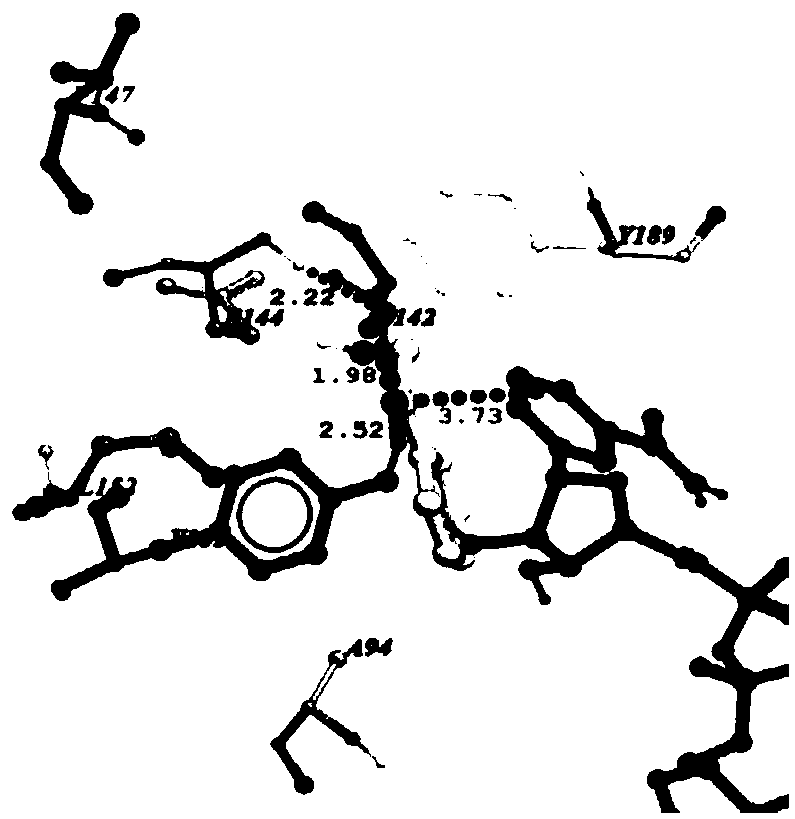
[0026] The changes between carbonyl reductase and 2-hydroxyacetophenone before and after mutation were analyzed by molecular docking; carbonyl reductase AcCR, mutant mut-AcCR (I147V / G152L) were docked with 2-hydroxyacetophenone respectively, and the enzymes were analyzed The distance between the active site Ser142, Tyr155 and the coenzyme NADH nicotinamide ring C4 located between the substrate and 2-hydroxyacetophenone and the change of the interaction force. Figure 1a , Figure 1b The docking results of AcCR and mut-E144A / G152L with 2-hydroxyacetophenone are shown. a, b are the docking results of 2-hydroxyacetophenone with AcCR and mut-I147V / G152L. from Figure 1a , Figure 1b It can be seen that after the mutation, the distance between the catalytic site S142 and the hydrogen atom at the 4-position of the NADH nicotinamide ring and the carbonyl oxygen atom of 4’-chloroacetophenone is significantly reduced, shortening respectively (20.1%) and (7.1%). The above results ...
Embodiment 3
[0028] The plasmid pGEX-mut-I147V / G152L containing the mutant mut-AcCR (I147V / G152L) gene was obtained by amplifying the whole pGEX-acr plasmid using PrimeSTAR Max DNA Polymerase.
[0029]Primers used for site-directed mutagenesis: the mutation primer at site I1447 is Primer1: 5'-ACTGGTAGGGGACCCAATGGGAGCCG-3'; ', Primer 4: 5'-GTTATAGGCGGCCAACATTGGGT-3'.
[0030] The PCR amplification system and reaction conditions used for site-directed mutagenesis are as follows:
[0031] Polymerase Chain Reaction (PCR) Amplification System
[0032]
[0033] PCR reaction conditions:
[0034]
[0035] After the reaction, the reaction product was treated with restriction endonuclease DpnI to act on the Gm6A^TC site to digest the template plasmid in the system. The reaction system is:
[0036]
[0037]
[0038] Place the prepared digestion reaction system at 37°C and incubate for 15 minutes to complete the digestion process of the template plasmid. The digested product was direc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com