Method for preparing high-purity ethylene glycol diacetate through direct esterification
A technology of ethylene glycol diacetate, which is applied in the field of preparing high-purity ethylene glycol diacetate, can solve the problems of affecting the purity of ethylene glycol diacetate, increasing the energy consumption of tower 3, and reducing the separation capacity. Achieve good economic and environmental benefits, increase economic costs, and achieve high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
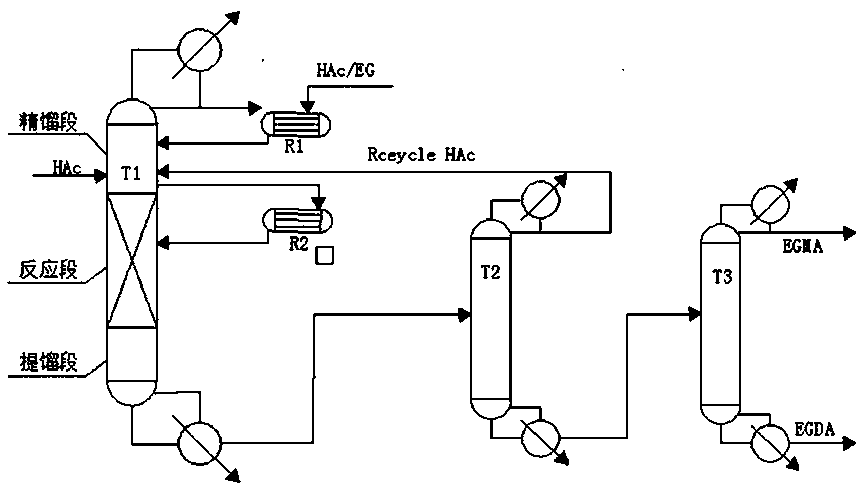
[0024] process such as figure 1As shown, the raw material ethylene glycol and acetic acid are preheated to 160°C with a molar ratio of 1:2.4, and the acetic acid feed is divided into two streams, one is fed from the fifth plate of T1, and the flow rate is 11.76 kmol / h, and The reflux ratio of T1 is 0.8, the molar ratio of output and feed is 0.53, another 58.8kmol / h acetic acid and 29.4kmol / h ethylene glycol are mixed into the knapsack reactor, and the raw materials are in the reactive distillation column (T1) The internal reverse flow is fully contacted, and all the liquid phase on the plate of the reactive distillation tower (T1) is withdrawn from the side line and enters the knapsack reactor for reaction, and the reacted mixture completely enters the reactive distillation tower (T1) for separation; The water produced by the reaction and a small amount of unreacted acetic acid enter the rectification section of the reactive distillation column (T1) for separation, and then a...
Embodiment 2
[0027] process such as figure 1 As shown, the raw material ethylene glycol and acetic acid are preheated to 160°C with a molar ratio of 1:2.6, and the acetic acid feed is divided into two streams, one is fed from the fifth plate of T1, and the flow rate is 16.6 kmol / h, and The reflux ratio of T1 is 1.1, the molar ratio of output and feed is 0.53, another 55.6kmol / h acetic acid and 27.8kmol / h ethylene glycol are mixed into the knapsack reactor, and the raw materials are in the reactive distillation column (T1) The internal reverse flow is fully contacted, and all the liquid phase on the plate of the reactive distillation tower (T1) is withdrawn from the side line and enters the knapsack reactor for reaction, and the reacted mixture completely enters the reactive distillation tower (T1) for separation; The water produced by the reaction and a small amount of unreacted acetic acid enter the rectification section of the reactive distillation column (T1) for separation, and then a...
Embodiment 3
[0030] process such as figure 1 As shown, the raw material ethylene glycol and acetic acid are preheated to 160°C with a molar ratio of 1:2.6, and the acetic acid feed is divided into two streams, one is fed from the fifth plate of T1, and the flow rate is 16.6 kmol / h, and The reflux ratio of T1 is 1.3, the molar ratio of output and feed is 0.53, another 55.6kmol / h acetic acid and 27.8kmol / h ethylene glycol are mixed into the knapsack reactor, and the raw materials are in the reactive distillation column (T1) The internal reverse flow is fully contacted, and all the liquid phase on the plate of the reactive distillation tower (T1) is withdrawn from the side line and enters the knapsack reactor for reaction, and the reacted mixture completely enters the reactive distillation tower (T1) for separation; The water produced by the reaction and a small amount of unreacted acetic acid enter the rectification section of the reactive distillation column (T1) for separation, and then a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com