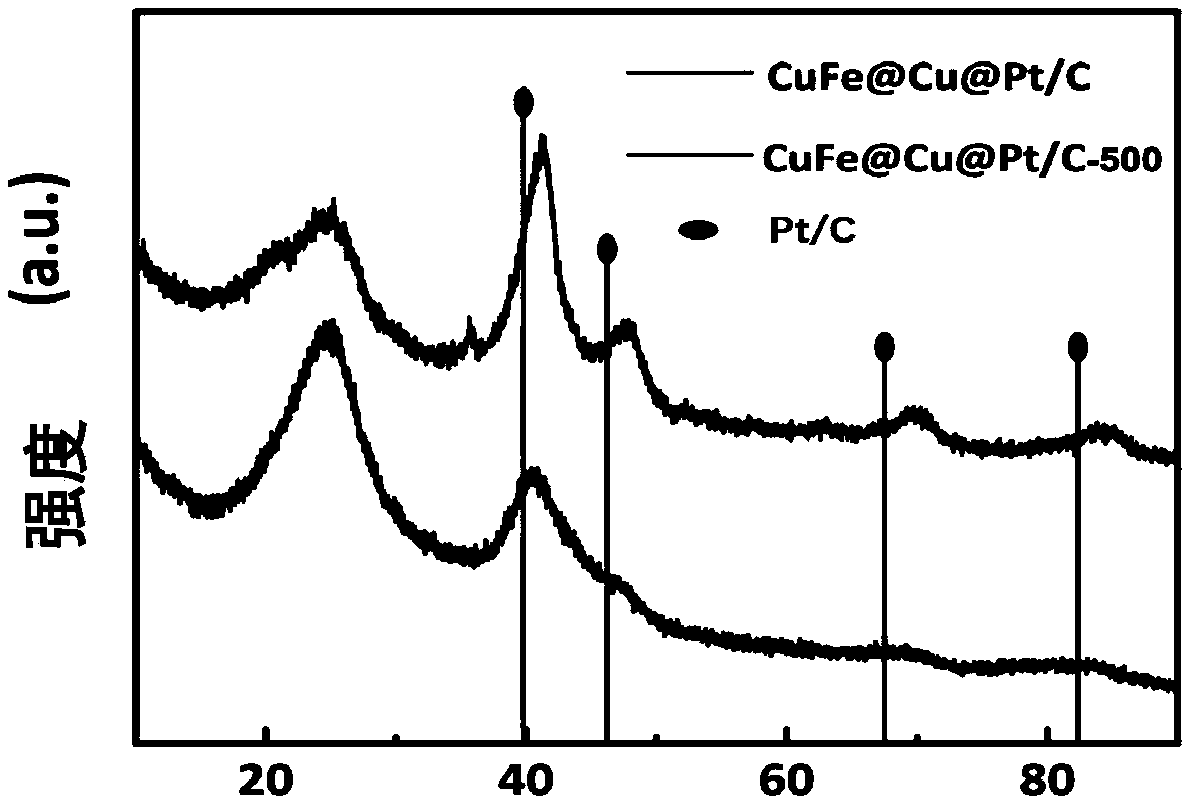
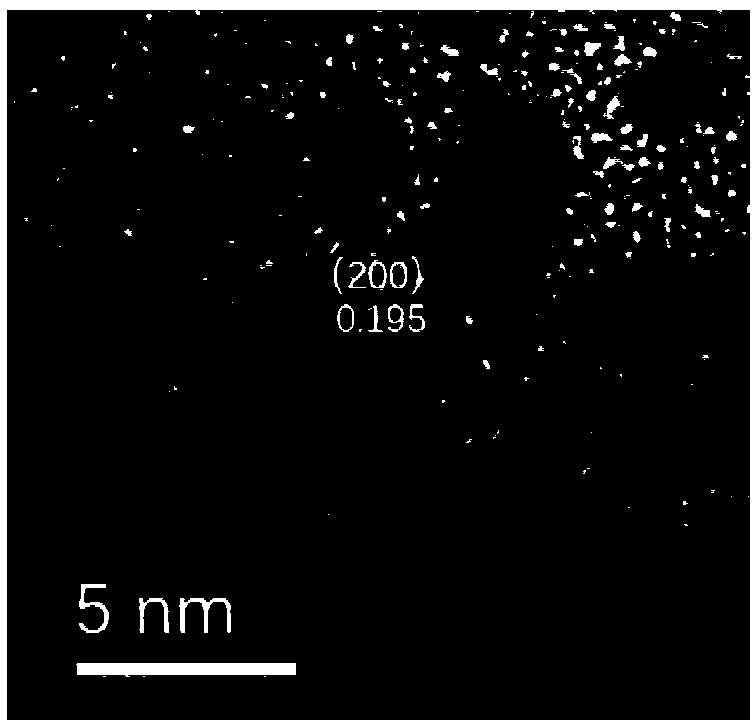
Method for preparing carbon-supported core-shell compact copper iron-copper-platinum catalyst for fuel cell
A fuel cell and platinum catalyst technology, applied in the field of electrochemistry, can solve the problems of inability to form lattice stress, expensive palladium, limited activity, etc., and achieve the effects of improving utilization and stability, promoting further development, and reducing preparation costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Weigh 80mg of activated carbon powder and place it in a conical flask, add 40ml of ethylene glycol (EG), ultrasonically disperse at room temperature for 1h, and then add soluble copper with a concentration of 20g / L under magnetic stirring Salt (analytical pure copper chloride or analytical pure copper nitrate) in ethylene glycol solution and iron salt (analytical pure ferric chloride or analytical pure ferric nitrate) in ethylene glycol solution, so that carbon, copper ions and iron ions in the mixed solution The mass ratio of 80:17:3, magnetic stirring 1h;
[0056] (2) Use 2M potassium hydroxide solution dissolved in ethylene glycol to adjust the pH of the above mixed solution to 10. After a period of stabilization, add 20 mL of ethylene glycol solution dropwise under nitrogen protection and strong stirring. Alcohol Sodium Borohydride (NaBH 4 , 2M) solution, the reaction time is 1h, to obtain carbon-loaded copper-iron alloy slurry;
[0057] (3) With the carbon-su...
Embodiment 2
[0062] (1) Weigh 70 mg of activated carbon powder and place it in a conical flask, add 40 ml of ethylene glycol (EG), ultrasonically disperse at room temperature for 1 h, and then add soluble copper with a concentration of 20 g / L under magnetic stirring Salt (analytical pure copper chloride or analytical pure copper nitrate) in ethylene glycol solution and iron salt (analytical pure ferric chloride or analytical pure ferric nitrate) in ethylene glycol solution, so that carbon, copper ions and iron ions in the mixed solution The mass ratio of 80:15:2, magnetic stirring 1h;
[0063] (2) Adjust the pH of the above mixed solution to 10 with a concentration of 2M potassium hydroxide solution dissolved in ethylene glycol. After a period of stability, under nitrogen protection and strong stirring, add 17ml of ethylene glycol solution dropwise. Alcohol Sodium Borohydride (NaBH 4 , 2M) solution, the reaction time is 1h, to obtain carbon-loaded copper-iron alloy slurry;
[0064] (3) W...
Embodiment 3
[0069] (1) Weigh 65g of activated carbon powder and place it in a conical flask, add 40ml of ethylene glycol (EG), ultrasonically disperse at room temperature for 1h, then add soluble copper with a concentration of 20g / L under magnetic stirring Salt (analytical pure copper chloride or analytical pure copper nitrate) in ethylene glycol solution and iron salt (analytical pure ferric chloride or analytical pure ferric nitrate) in ethylene glycol solution, so that carbon, copper ions and iron ions in the mixed solution The mass ratio of 70:15:3, magnetic stirring 1h;
[0070] (2) Use 2M potassium hydroxide solution dissolved in ethylene glycol to adjust the pH of the above mixed solution to 10. After a period of stability, under nitrogen protection and strong stirring, add 18 mL of ethylene glycol solution dropwise. Alcohol Sodium Borohydride (NaBH 4 , 2M) solution, the reaction time is 1h, to obtain carbon-loaded copper-iron alloy slurry;
[0071] (3) With the carbon-supported ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com