Synthesis of Dapagliflozin
A synthesis method and technology of mixed solutions, applied in the fields of organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of long reaction time and high energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
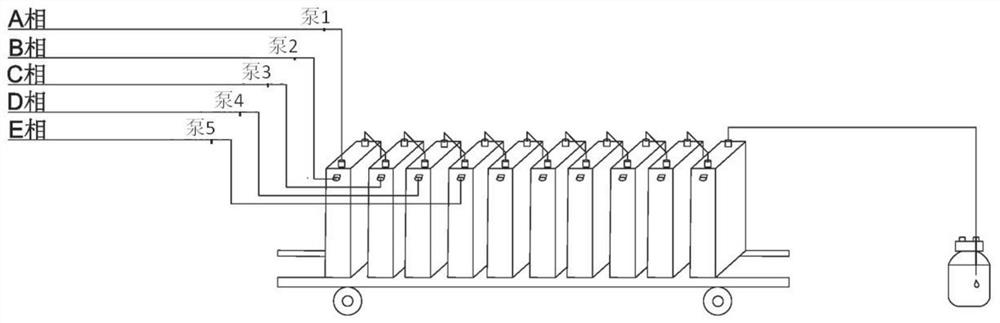
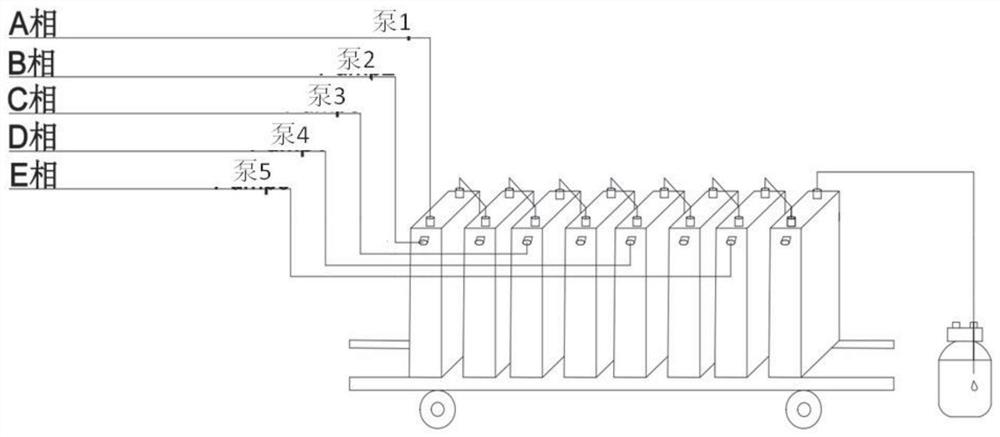
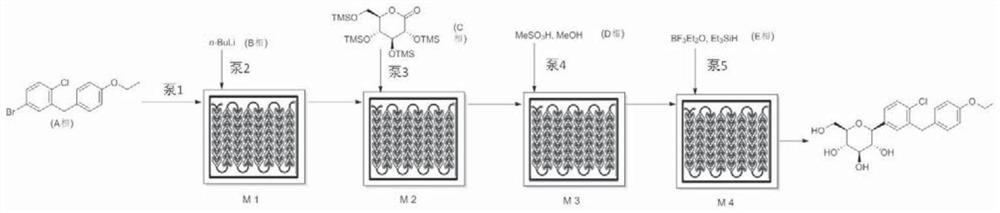
[0084] Accurately weigh 65.12g (0.2Mol) raw material A (5-bromo-2-chloro-4'-ethoxydiphenylmethane, the same below), then add toluene / tetrahydrofuran mixed solution (V 甲苯 / 四氢呋喃 = 2: 1), set the volume to 500ml, pump it into the first reaction unit of the microreactor with a flow rate of 13ml / min after mixing, and simultaneously use a concentration of 2.0mol / L n-butyllithium solution to pump Into the first reaction unit for full mixing and lithium halogen exchange reaction, the residence time is 30 seconds, the reaction temperature is controlled at -25 ° C, after the reaction liquid flows out of the first reaction unit, the raw material C (trimethylsilyl-protected Gluconolactone, the same below) flows into the second reaction unit of the microreactor with a flow rate of 2ml / min to carry out the lactone condensation reaction. The residence time of this step reaction is 24 seconds, and the reaction temperature of the control second reaction unit is -25 ℃.
[0085] Accurately prepa...
Embodiment 2
[0088] Accurately weigh 65.12g (0.2Mol) raw material A, then add toluene / tetrahydrofuran mixed solution (V 甲苯 / 四氢呋喃 =2:1), set the volume to 500ml, pump it into the first reaction unit of the microreactor with a flow rate of 14ml / min after mixing, and simultaneously inject the concentration of 2.0mol / L n-butyllithium solution with a flow rate of 3.4ml / min Pump into the first reaction unit for thorough mixing to generate lithium-halide exchange reaction. The residence time is 27 seconds. The reaction temperature is controlled at -25°C. The second reaction unit flowing into the microreactor performs lactone condensation reaction, the residence time of this step reaction is 22 seconds, and the reaction temperature of the second reaction unit is controlled to be -25°C.
[0089] Accurately prepare the mixed solution of 300ml methanol and methanesulfonic acid (V 甲醇 / 甲烷磺酸 =4: 1), with the flow rate of 3.5ml / min, it is transported into the third reaction unit of the microreactor, mixed...
Embodiment 3
[0092] Accurately weigh 65.12g (0.2Mol) raw material A, then add toluene / tetrahydrofuran mixed solution (V 甲苯 / 四氢呋喃 =2:1), set the volume to 500ml, pump it into the first reaction unit of the microreactor with a flow rate of 14ml / min after mixing, and simultaneously inject the concentration of 2.0mol / L n-butyllithium solution with a flow rate of 3.4ml / min Pump into the first reaction unit for thorough mixing to generate lithium-halide exchange reaction. The residence time is 27 seconds. The reaction temperature is controlled at -28°C. The second reaction unit flowing into the microreactor performs lactone condensation reaction, the residence time of this step reaction is 21 seconds, and the reaction temperature of the second reaction unit is controlled to be -28°C.
[0093] Accurately prepare the mixed solution of 300ml methanol and methanesulfonic acid (V 甲醇 / 甲烷磺酸 =4: 1), with the flow rate of 3.5ml / min, it is transported into the third reaction unit of the microreactor, mixed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com