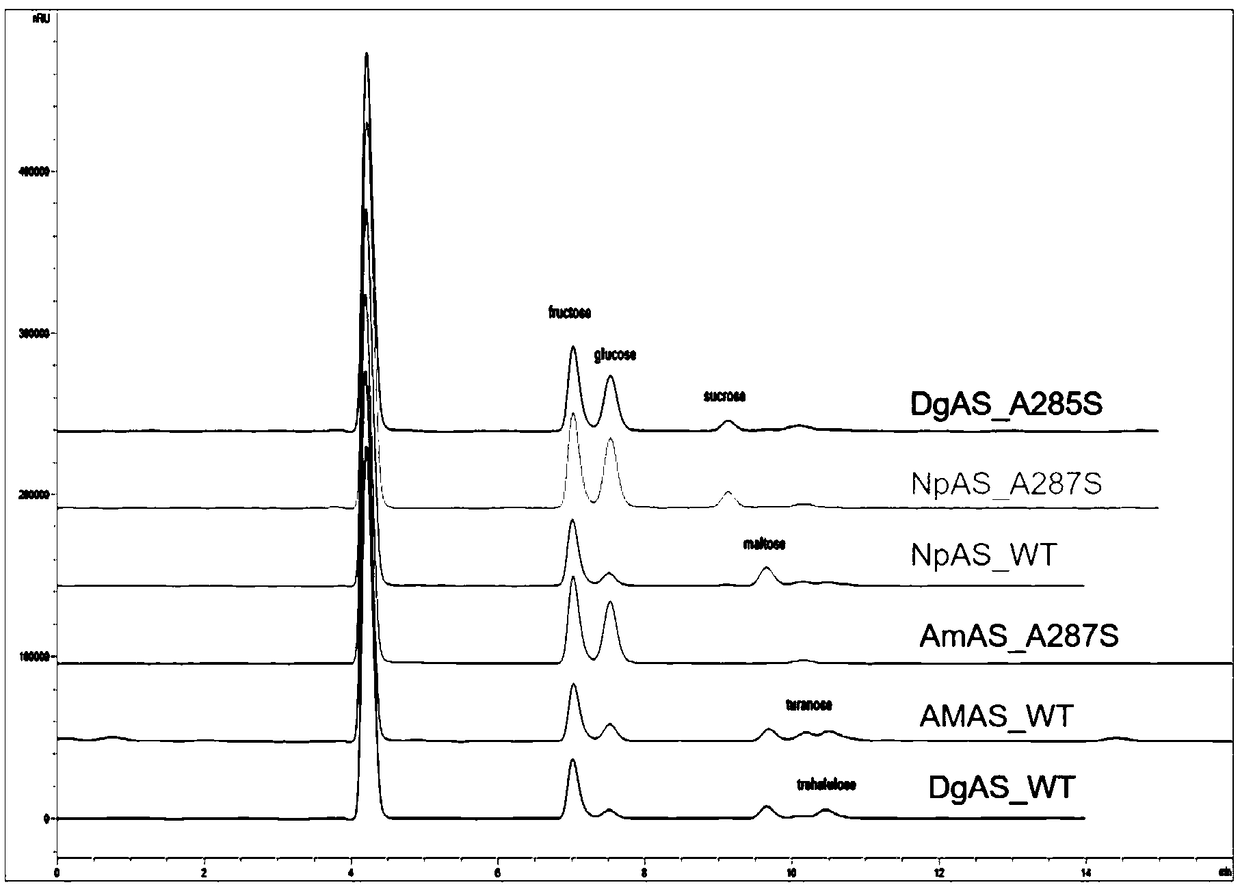
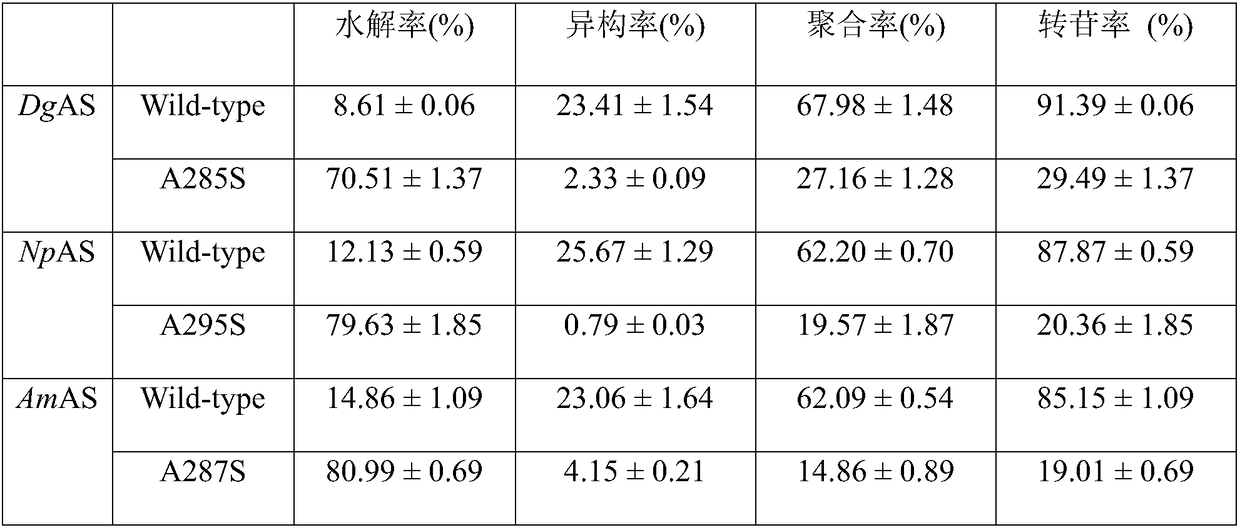
Amylosucrase mutant and preparation method and application thereof
A technology of amylosucrase and mutants, applied in the fields of genetic engineering and enzyme engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Recombinant bacteria construction
[0035] According to the amylosucrase gene sequence with accession number ABF44874.1 on NCBI, the DgAS gene containing amylosucrase was synthesized by chemical synthesis, and the amylosucrase gene sequence with accession number Q9ZEU2.1 was synthesized by chemical synthesis. NpAS gene, accession number BAG82876.1 Amylosucrase gene sequence Synthesize AmAS gene containing amylosucrase by chemical synthesis. The DgAS gene, NpAS gene, AmAS gene and the pET-24a(+) plasmid were double-digested with NdeI and HindIII respectively, and the digested products were recovered by tapping the rubber, and then ligated with T4 ligase, and the ligated products were transformed into E.coliJM109 competent cells. Obtain recombinant cells. The recombinant cells were cultured at 37°C for 8 hours, and the transformants were picked and cultured with shaking in LB liquid medium (containing 30 mg / L kanamycin), the plasmids were extracted, and the...
Embodiment 2
[0038] Embodiment 2: the preparation of amylosucrase mutant
[0039] (1) Preparation of amylosucrase single mutation
[0040] According to the DgAS gene sequence of amylosucrase, the primers for introducing the A285S mutation were designed and synthesized. Using rapid PCR technology, the plasmid DgAS / pET-24a(+) carrying the gene encoding wild-type amylosucrase was used as a template to target amylosucrase. The DgAS gene sequence was subjected to site-directed mutation, and the DNA coding sequence was determined to identify the gene in which the 285th Ala codon was changed to a Ser codon, and amylosucrase single mutation A285S was obtained.
[0041] According to the AmAS gene sequence of amylosucrase, the primers for introducing the A287S mutation were designed and synthesized, and the plasmid AmAS / pET-24a(+) carrying the gene encoding wild-type amylosucrase was used as a template by using rapid PCR technology to target amylosucrase. The AmAS gene sequence was subjected to sit...
Embodiment 3
[0064] Embodiment 3: the concentration of crude enzyme liquid
[0065] Slowly add ammonium sulfate with a concentration of 20% relative to the mass fraction of the enzyme liquid while stirring the crude enzyme liquid obtained in Examples 1 and 2, stir until the ammonium sulfate is dissolved, and stand at 4°C for 8 to 10 hours to precipitate protein . The mixture was centrifuged (8000rpm, 10min) to collect the precipitate, and the minimum volume of 50mM KH 2 PO 4 -Na 2 HPO 4 The buffer (pH 7.0) was redissolved, and after reconstitution, the solid matter was removed by centrifugation again, and the supernatant was collected and dialyzed to obtain a concentrated enzyme solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com